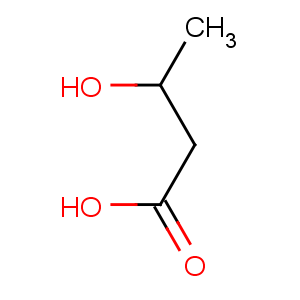
Title: b-Hydroxybutyric Acid
CAS Registry Number: 300-85-6
CAS Name: 3-Hydroxybutanoic acid
Molecular Formula: C4H8O3
Molecular Weight: 104.10
Percent Composition: C 46.15%, H 7.75%, O 46.11%
Line Formula: CH3CHOHCH2COOH
Derivative Type: d-Form
Literature References: Prepd by the action of
Aspergillus griseus on the
dl-form: McKenzie, Harden,
J. Chem. Soc. 83, 430 (1903).
Properties: Crystals. [a]D10 +24.3° (c = 2.226). Sol in water, alcohol, ether.
Optical Rotation: [a]D10 +24.3° (c = 2.226)
Derivative Type: l-Form
Literature References: Found in the urine of diabetics (as much as 30 g per day). Isoln: Fischer, Scheibler,
Ber. 42, 1221 (1909); Shaffer, Marriott,
J. Biol. Chem. 16, 268 (1913).
Properties: Hygroscopic, monoclinic crystals, mp 45.5-48°. [a]D25 -24.5° (c = 5). K at 22° = 3.86′10-5. Freely sol in water, alcohol, ether. Sparingly sol in benzene. On distn it dec into crotonic acid and water.
Melting point: mp 45.5-48°
Optical Rotation: [a]D25 -24.5° (c = 5)
Derivative Type: dl-Form
Literature References: Prepd from acetoacetic ester by the action of sodium amalgam: Wislicenus,
Ann. 149, 207 (1869); Marian,
Biochem. Z. 150, 283 (1924); by the oxidation of aldol: Wurtz,
Compt. Rend. 76, 1167 (1873); from crotonic acid by heating with dil acid: Wacker,
DE 441003;
Frdl. 15, 135; by heating crotonitrile with KOH soln: Bruylants,
Bull. Soc. Chim. Belg. 31, 182 (1922).
Properties: Hygroscopic syrup. Volatile with steam. Sol in water, alcohol, ether. On distn it dec into crotonic acid and water.