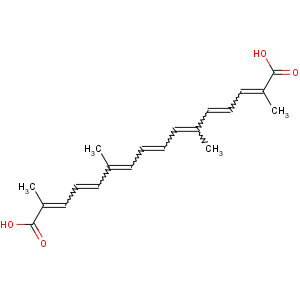
References of (2E,4E,6E,8E,10E,12E,14E)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,
14-heptaenedioic acid
Title: Crocetin
CAS Registry Number: 27876-94-4
CAS Name: 8,8¢-Diapo-y,y-carotenedioic acid
Synonyms: trans-crocetin
Molecular Formula: C20H24O4
Molecular Weight: 328.40
Percent Composition: C 73.15%, H 7.37%, O 19.49%
Literature References: Carotenoid-dicarboxylic acid isolated from
Crocus sativus L.;
C. albiflorus var.
neapolitanus Hort.;
C. luteus Lam.,
Iridaceae. Extraction procedure and structure: Jucker, Karrer,
Carotinoide (Basel, 1948) p 282.
Properties: Brick-red rhombs from acetic anhydride, mp 285°. Absorption max (pyridine): 464, 436, 411 nm. Sol in pyridine, in very dil NaOH solns. Very sparingly sol in water, and in the usual organic solvents except pyridine and similar organic bases. Forms a solid dipyridyl salt.
Melting point: mp 285°
Absorption maximum: Absorption max (pyridine): 464, 436, 411 nm
Derivative Type: Dimethyl ester
Molecular Formula: C22H28O4
Molecular Weight: 356.46
Percent Composition: C 74.13%, H 7.92%, O 17.95%
Properties: Brick-red elongated leaflets, mp 222.5°. Total synthesis: Buchta, Andree,
Naturwissenschaften 46, 74 (1959).
Melting point: mp 222.5°
Derivative Type: Di-gentiobiose ester
CAS Registry Number: 42553-65-1
CAS Name: Crocin
Molecular Formula: C44H64O24
Molecular Weight: 976.96
Percent Composition: C 54.09%, H 6.60%, O 39.30%
Literature References: One of the yellow-red pigments of saffron. Isoln and structure: Karrer, Salomon,
Helv. Chim. Acta 10, 397 (1927);
11, 513, 711 (1928); Karrer
et al., ibid. 12, 985 (1929);
13, 392 (1930); Kuhn, Winterstein,
Ber. 67, 344 (1934); Reichstein,
Angew. Chem. 74, 887 (1962).
Properties: Hydrated brownish-red needles from methanol, mp 186° (effervescence). Absorption max (methanol): 464, 434 nm. Freely sol in hot water giving an orange-colored soln. Sparingly sol in abs alcohol, ether, other organic solvents.
Melting point: mp 186° (effervescence)
Absorption maximum: Absorption max (methanol): 464, 434 nm