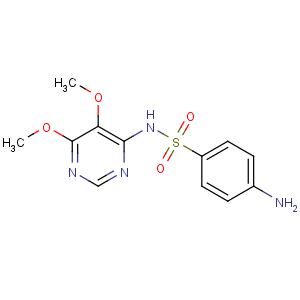
Title: Sulfadoxine
CAS Registry Number: 2447-57-6
CAS Name: 4-Amino-
N-(5,6-dimethoxy-4-pyrimidinyl)benzenesulfonamide
Synonyms: N¢-(5,6-dimethoxy-4-pyrimidinyl)sulfanilamide; 6-(4-aminobenzenesulfonamido)-4,5-dimethoxypyrimidine; 4-sulfanilamido-5,6-dimethoxypyrimidine; sulforthomidine; sulphormethoxine
Manufacturers' Codes: Ro-4-4393
Trademarks: Fanasil (Roche)
Molecular Formula: C12H14N4O4S
Molecular Weight: 310.33
Percent Composition: C 46.44%, H 4.55%, N 18.05%, O 20.62%, S 10.33%
Literature References: Dihydropteroate synthetase inhibitor. Prepn:
BE 618639; H. Bretschneider
et al., US 3132139 (1962, 1964 both to Hoffmann-La Roche). Toxicological, chemotherapeutic and pharmacokinetic studies: E. B?hni
et al., Chemotherapy 14, 195-226 (1969). Comprehensive description: V. K. Kapoor,
Anal. Profiles Drug Subs. 17, 571-605 (1988). Enzyme inhibition
in vitro: Y. Zhang, S. R. Meshnick,
Antimicrob. Agents Chemother. 35, 267 (1991). Determn in plasma using supercritical fluid chromatography: S. I. Bhoir
et al., J. Chromatogr. B 757, 39 (2001). Clinical trial of combination with pyrimethamine,
q.v., for malaria: C. V. Plowe
et al.,
Br. Med. J. 328, 545 (2004).
Properties: Crystals from 50% aq alc, mp 190-194°. Practically insol in ether. Very slightly sol in water, slightly sol in alcohol, methanol. Sol in dilute mineral acids, solutions of alkali hydroxides and carbonates. LD50 in mice (microcrystals, mg/kg): 5200 orally, 2900 s.c., 2900 i.p. (B?hni).
Melting point: mp 190-194°
Toxicity data: LD50 in mice (microcrystals, mg/kg): 5200 orally, 2900 s.c., 2900 i.p. (B?hni)
Derivative Type: Mixture with trimethoprim
CAS Registry Number: 39295-60-8
Trademarks: Animar (Pfizer); Bimotrim (Vetpharma); Borgal (Intervet)
Therap-Cat: Antibacterial; antimalarial.
Therap-Cat-Vet: Antibacterial.
Keywords: Antibacterial (Synthetic); Sulfonamides; Antimalarial.