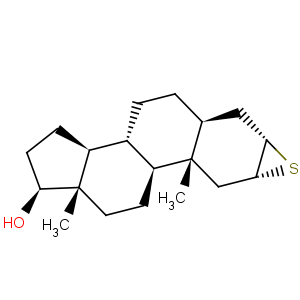
Title: Epitiostanol
CAS Registry Number: 2363-58-8
CAS Name: (2a,3a,5a,17b)-2,3-Epithioandrostan-17-ol
Manufacturers' Codes: 10275-S
Trademarks: Thiodrol (Shionogi)
Molecular Formula: C19H30OS
Molecular Weight: 306.51
Percent Composition: C 74.45%, H 9.87%, O 5.22%, S 10.46%
Literature References: Episulfide deriv of androstane,
q.v. Prepn:
GB 977599; T. Komeno,
US 3230215 (1964, 1966 both to Shionogi); K. Takeda
et al., Tetrahedron 1965, 329; P. D. Klimstra
et al., J. Med. Chem. 9, 693 (1966). Antitumor effect in mice: A. Matsuzawa, T. Yamamoto,
Cancer Res. 37, 4408 (1977). Teratogenicity study: T. Minesita
et al., Oyo Yakuri 7, 723 (1973),
C.A. 80, 116474p (1974). Toxicity study:
eidem, ibid. 805,
C.A. 80, 66865u (1974). Use in treatment of breast cancer: M. Fujimoro
et al., Cancer 31, 789 (1973).
Properties: Crystals from acetone, mp 127-128°. [a]D27.5 +24.4° (c = 1.054 in chloroform). uv max (alcohol): 262 nm. LD50 in mice, rats (mg/kg): 1, 5 i.p. (Minesita, p 805).
Melting point: mp 127-128°
Optical Rotation: [a]D27.5 +24.4° (c = 1.054 in chloroform)
Absorption maximum: uv max (alcohol): 262 nm
Toxicity data: LD50 in mice, rats (mg/kg): 1, 5 i.p. (Minesita, p 805)
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic (Hormonal); Androgens.