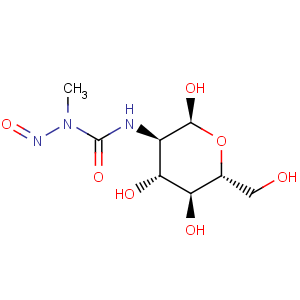
Title: Streptozocin
CAS Registry Number: 18883-66-4
CAS Name: 2-Deoxy-2-[[(methylnitrosoamino)carbonyl]amino]-D-glucopyranose
Synonyms: 2-deoxy-2-(3-methyl-3-nitrosoureido)-D-glucopyranose; streptozotocin
Manufacturers' Codes: NSC-85998; U-9889
Trademarks: Zanosar (Pharmacia & Upjohn)
Molecular Formula: C8H15N3O7
Molecular Weight: 265.22
Percent Composition: C 36.23%, H 5.70%, N 15.84%, O 42.23%
Literature References: Isoln from
Streptomyces achromogenes fermentation broth: Herr
et al., Antibiot. Annu. 1959-1960, 236; Bergy
et al., FR 1434920 (1966 to Upjohn),
C.A. 65, 17661h (1966). Structure and synthesis: Herr
et al., J. Am. Chem. Soc. 89, 4808 (1967); Hardegger
et al., Helv. Chim. Acta 52, 2555 (1969); Hessler, Jahnke,
J. Org. Chem. 35, 245 (1970); P. F. Wiley
et al., ibid. 44, 9 (1979). Diabetogenic effect: Rakieten
et al., Cancer Chemother. Rep. 29, 91 (May 1963). Comparison of streptozotocin- and alloxan-induced diabetes: C. Rerup, F. Tarding,
Eur. J. Clin. Pharmacol. 7, 89 (1969). Antileukemic activity: Bhuyan
et al., Cancer Chemother. Rep. Part 1 56, 709 (1972). Biosynthetic study: S. Singaram
et al., J. Antibiot. 32, 379 (1979). Review and evaluation of studies of carcinogenicity in laboratory animals:
IARC Monographs 4, 221-227 (1974). Toxicity data: M. Iwasaki
et al., J. Med. Chem. 19, 918 (1976).
Review: B. Rudas,
Arzneim.-Forsch. 22, 830-861 (1972); P. F. Wiley,
Anticancer Agents Based on Natural Product Models, J. M. Cassidy, J. D. Douros, Eds. (Academic Press, New York, 1980) pp 167-200. Book:
Streptozotocin: Fundamentals and Therapy, M. K. Agarwal, Ed. (Elsevier/North Holland Biomedical Press, New York, 1981) 309 pp.
Properties: Pointed platelets or prisms, from 95% ethanol, mp 115° (dec). Sol in H2O, lower alcohols and ketones. uv max (ethanol): 228 nm (e 6360). A mixture of a and b anomers; aq solns rapidly undergo mutarotation to an equilibrium value of [a]D25 +39°. LD50 in female mice (mg/kg): 360 i.p.; 275 i.v.; in male dogs (mg/kg): 50 i.v. (Iwasaki).
Melting point: mp 115° (dec)
Optical Rotation: [a]D25 +39°
Absorption maximum: uv max (ethanol): 228 nm (e 6360)
Toxicity data: LD50 in female mice (mg/kg): 360 i.p.; 275 i.v.; in male dogs (mg/kg): 50 i.v. (Iwasaki)
Derivative Type: Tetraacetate
Molecular Formula: C8H11N3O7(COCH3)4
Molecular Weight: 433.37
Percent Composition: C 44.34%, H 5.35%, N 9.70%, O 40.61%
Properties: Crystals, mp 111-114° (dec). [a]D25 +41° (c = 0.78 in 95% ethanol).
Melting point: mp 111-114° (dec)
Optical Rotation: [a]D25 +41° (c = 0.78 in 95% ethanol)
CAUTION: Streptozocin is reasonably anticipated to be a human carcinogen:
Report on Carcinogens, Eleventh Edition (PB2005-104914, 2004) p III-234.
Use: Production of experimental diabetes in laboratory animals.
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic; Antibiotics and Analogs.