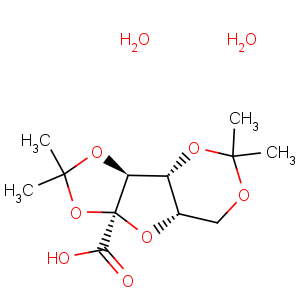
References of a-L-xylo-2-Hexulofuranosonic acid,2,3:4,6-bis-O-(1-methylethylidene)-
Title: Dikegulac
CAS Registry Number: 18467-77-1
CAS Name: 2,3:4,6-Bis-
O-(methylethylidene)-a-L-
xylo-2-hexulofuranosonic acid
Synonyms: a-2,3:4,6-di-
O-isopropylidene-L-
xylo-hexulofuranosonic acid; di-
O-isopropylidene-2-keto-L-gulonic acid; diacetone-2-ketogulonic acid; diacetone-2-oxo-L-gulonic acid; oxogulonic acid diacetonide
Molecular Formula: C12H18O7
Molecular Weight: 274.27
Percent Composition: C 52.55%, H 6.61%, O 40.83%
Literature References: Intermediate in the manuf of ascorbic acid,
q.v. Manufacturing process: G. M. Jaffe, E. J. Pleven,
DE 2123621;
eidem, US 3832355 (1970, 1974 both to Hoffmann-La Roche). Use as a plant growth regulator: W. Szkrybalo,
DE 2339239;
eidem, US 4337080 (1974, 1982 both to Hoffmann-La Roche); P. Bocian
et al., Nature 258, 142 (1975). Physicochemical properties, toxicity and growth retardant effect: W. H. de Silva
et al., Proc. Br. Crop Prot. Conf. - Weeds 1976, 349. Activity: S. S. Purohit,
Comp. Physiol. Ecol. 4, 264 (1979);
6, 261 (1981).
Derivative Type: Sodium salt
Synonyms: Dikegulac-sodium
Manufacturers' Codes: Ro-7-6145
Trademarks: Atrinal (Roche)
Molecular Formula: C12H17NaO7
Molecular Weight: 296.25
Percent Composition: C 48.65%, H 5.78%, Na 7.76%, O 37.80%
Properties: Powder, mp >300°. Vapor pressure at 25°: <10-10 mm Hg. Soly at 20° (g/l): water 590; methanol 390; ethanol 230; chloroform 60; acetone <10; hexane <10; cyclohexanone <10. LD50 in mice, male, female rats (mg/kg): 19500, 31000, 18000 orally; LC50 (96 hr) in bluegill sunfish, rainbow trout (ppm): >10000, >5000 (de Silva).
Melting point: mp >300°
Toxicity data: LD50 in mice, male, female rats (mg/kg): 19500, 31000, 18000 orally; LC50 (96 hr) in bluegill sunfish, rainbow trout (ppm): >10000, >5000 (de Silva)
Use: Plant growth regulator; herbicide.