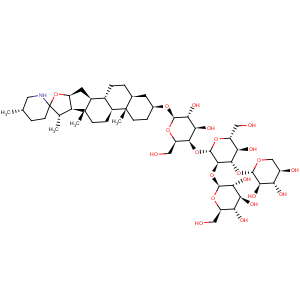
Title: Tomatine
CAS Registry Number: 17406-45-0
CAS Name: (3b,5a,22b,25
S)-Spirosolan-3-yl
O-b-D-glucopyranosyl-(1?2)-
O-[b-D-xylopyranosyl-(1?3)]-
O-b-D-glucopyranosyl-(1?4)-b-D-galactopyranoside
Synonyms: lycopersicin
Molecular Formula: C50H83NO21
Molecular Weight: 1034.19
Percent Composition: C 58.07%, H 8.09%, N 1.35%, O 32.49%
Literature References: Occurs in the extract of leaves of wild tomato plants: Fontaine
et al., Arch. Biochem. 18, 467 (1948); Kuhn, Low,
Ber. 81, 552 (1948); Kuhn
et al., ibid. 83, 448 (1950); Bognar, Makleit,
Pharmazie 11, 376 (1956). Yields on partial hydrolysis, besides a-tomatine, the main constituent, b1-, b2-, g- and d-tomatine: Kuhn
et al., Ber. 90, 203 (1957). a-Tomatine consists of one mol tomatidine linked to a tetrasaccharide composed of 2 mols D-glucose, 1 mol D-xylose and 1 mol D-galactose: Kuhn
et al., Angew. Chem. 68, 212 (1956). Proposed as an alternate precipitant to digitonin: Schultz, Sander,
Z. Physiol. Chem. 308, 122 (1957). Structure: Reichstein,
ibid. 74, 887 (1962). Toxicity study: Wilson
et al., Toxicol. Appl. Pharmacol. 3, 39 (1961).
Properties: Needles from methanol, mp 263-268°. [a]D20 -18° (c = 0.55 in pyridine). Sol in ethanol, methanol, dioxane, propylene glycol. Practically insol in water, ether, petr ether. Stable to strong alkali but hydrolyzed by acids to produce cryst tomatidine and a soln rich in reducing sugars. Has been found to inhibit the growth of various fungi and bacteria. LD orally in rats: 900-1000 mg/kg (Wilson).
Melting point: mp 263-268°
Optical Rotation: [a]D20 -18° (c = 0.55 in pyridine)
Toxicity data: LD orally in rats: 900-1000 mg/kg (Wilson)
Use: Precipitating agent for steroids.