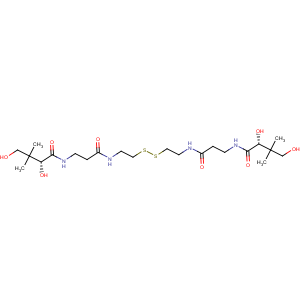
Title: Pantethine
CAS Registry Number: 16816-67-4
CAS Name: N,N¢-[Dithiobis[2,1-ethanediylimino(3-oxo-3,1-propanediyl)]]bis[2,4-dihydroxy-3,3-dimethylbutanamide]
Synonyms: N,N¢-[dithiobis(ethyleneiminocarbonylethylene)]bis(2,4-dihydroxy-3,3-dimethylbutyramide); D-bis(
N-pantothenyl-b-aminoethyl) disulfide
Trademarks: Lipodel (Delalande); Pantetina (Maggioni); Panthecin (Sawai); Pantomin (Daiichi); Pantosin (Daiichi)
Molecular Formula: C22H42N4O8S2
Molecular Weight: 554.72
Percent Composition: C 47.63%, H 7.63%, N 10.10%, O 23.07%, S 11.56%
Line Formula: [HOCH2C(CH3)2CHOHCONHCH2CH2CONHCH2CH2S]2
Literature References: Disulfide dimer of pantetheine,
q.v. Growth factor for
Lactobacillus bulgaricus: Williams
et al., J. Biol. Chem. 177, 933 (1949). Formed by oxidation of pantetheine: Brown, Snell,
J. Biol. Chem. 198, 375 (1952). Structure: Snell
et al., J. Am. Chem. Soc. 72, 5349 (1950). Synthesis: Wieland, Bokelmann,
Naturwissenschaften 38, 384 (1951); Wittle
et al., J. Am. Chem. Soc. 75, 1694 (1953); Viscontini
et al., Helv. Chim. Acta 37, 375 (1954); Bowman, Cavalla,
J. Chem. Soc. 1954, 1171; Shimizu
et al., Chem. Pharm. Bull. 13, 180 (1965). Clinical trial in hyperlipoproteinemia: A. Gaddi
et al., Atherosclerosis 50, 73 (1984).
Properties: Glassy, colorless to light yellow substance. [a]D27 +13.5° (c = 3.75 in water). Freely sol in water; less sol in ethanol. Practically insol in ether, acetone, ethyl acetate, benzene, chloroform.
Optical Rotation: [a]D27 +13.5° (c = 3.75 in water)
Therap-Cat: Antilipemic.
Keywords: Antilipemic.