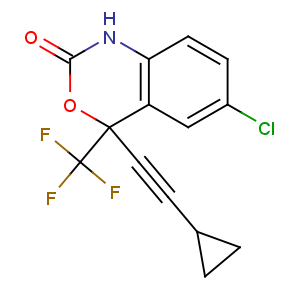
Title: Efavirenz
CAS Registry Number: 154598-52-4
CAS Name: (4
S)-6-Chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2
H-3,1-benzoxazin-2-one
Manufacturers' Codes: DMP-266
Trademarks: Stocrin (Merck & Co.); Sustiva (BMS)
Molecular Formula: C14H9ClF3NO2
Molecular Weight: 315.67
Percent Composition: C 53.27%, H 2.87%, Cl 11.23%, F 18.06%, N 4.44%, O 10.14%
Literature References: Nonnucleoside HIV-1 reverse transcriptase inhibitor. Prepn: S. D. Young
et al., EP 582455;
eidem, US 5519021 (1994, 1996 both to Merck & Co.). Pharmacology: S. D. Young
et al., Antimicrob. Agents Chemother. 39, 2602 (1995). Enantioselective synthesis: A. S. Thompson
et al., Tetrahedron Lett. 36, 8937 (1995). Total synthesis and resolution of enantiomers: L. A. Radesca
et al., Synth. Commun. 27, 4373 (1997). Ionization behavior: S. R. Rabel
et al., Pharm. Dev. Technol. 1, 91 (1996). Clinical trial as component of combination therapy in HIV-1 infection in adults: S. Staszewski
et al., N. Engl. J. Med. 341, 1865 (1999); in children: S. E. Starr
et al., ibid. 1874. Review of clinical experience: C. Fortin, V. Joly,
Expert Rev. Anti Infect. Ther. 2, 671-684 (2004).
Properties: Crystals from toluene:heptane, mp 139-141°. [a]D20 -84.7° (c = 0.005 g/ml in CH3Cl). [a]D25 -94.1° (c = 0.300 in methanol). pKa 10.2.
Melting point: mp 139-141°
pKa: pKa 10.2
Optical Rotation: [a]D20 -84.7° (c = 0.005 g/ml in CH3Cl); [a]D25 -94.1° (c = 0.300 in methanol)
Therap-Cat: Antiviral.
Keywords: Antiviral; Reverse Transcriptase Inhibitor.