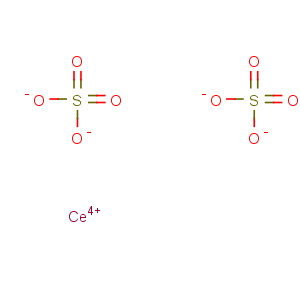Title: Ceric Sulfate
CAS Registry Number: 13590-82-4
Molecular Formula: CeO8S2
Molecular Weight: 332.24
Percent Composition: Ce 42.17%, O 38.52%, S 19.30%
Line Formula: Ce(SO4)2
Literature References: Prepd by heating CeO2 with concd H2SO4: Vanino,
Handbuch der Pr?parativen Chemie vol. 1 (Stuttgart, 3rd ed., 1925) p 755.
Review: Smith,
Ceric Sulfate (G. Frederick Smith Chem. Co., Columbus, Ohio, 2nd ed., 1935) 51 pp. The commercial salt usually contains small amounts of associated rare earths.
Derivative Type: Tetrahydrate
Properties: Yellow to orange powder or orthorhombic crystals. On heating loses water, becoming anhydr at 180-200°; dec above 350°, forming CeOSO4. Sol in a small quantity of water, but dec with much water with separation of a basic salt. Slowly sol in cold, more readily sol in hot mineral acids; sol in dil H2SO4.
Use: Oxidizing agent; analytical reagent; in radiation dosimeters.