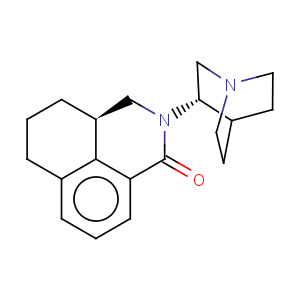
Title: Palonosetron
CAS Registry Number: 135729-61-2
CAS Name: (3a
S)-2-(3
S)-1-Azabicyclo[2.2.2]oct-3-yl-2,3,3a,4,5,6-hexahydro-1
H-benz[
de]isoquinolin-1-one
Molecular Formula: C19H24N2O
Molecular Weight: 296.41
Percent Composition: C 76.99%, H 8.16%, N 9.45%, O 5.40%
Literature References: Serotonin 5-HT3 receptor antagonist. Prepn: J. Berger
et al., EP 430190;
eidem,
US 5202333 (1991, 1993 both to Syntex); R. D. Clark
et al., J. Med. Chem. 36, 2645 (1993). Improved synthesis: B. A. Kowalczyk,
Heterocycles 43, 1439 (1996). Receptor binding study: E. H. F. Wong
et al., Br. J. Pharmacol. 114, 851 (1995). Pharmacology: R. M. Eglen
et al., ibid. 860. Clinical efficacy and pharmacokinetics: P. Eisenberg
et al., Ann. Oncol. 15, 330 (2004). Clinical trial in prevention of chemotherapy-induced nausea and vomiting: R. Gralla
et al., Ann. Oncol. 14, 1570 (2003). Review of pharmacology and clinical experience: S. M. Grunberg, J. M. Koeller,
Expert. Opin. Pharmacother. 4, 2297-2303 (2003); M. A. A. Siddiqui, L. J. Scott,
Drugs 64, 1125-1132 (2004).
Properties: mp 87-88°. [a]D -136° (c = 0.25 in chloroform).
Melting point: mp 87-88°
Optical Rotation: [a]D -136° (c = 0.25 in chloroform)
Derivative Type: Hydrochloride
CAS Registry Number: 135729-62-3
Manufacturers' Codes: RS-25259-197
Trademarks: Aloxi (Helsinn); Onicit (Helsinn)
Molecular Formula: C19H24N2O.HCl
Molecular Weight: 332.87
Percent Composition: C 68.56%, H 7.57%, N 8.42%, O 4.81%, Cl 10.65%
Properties: Crystals from ethanol, mp >290°. [a]D25 -94.1° (c = 0.4 in water). Freely sol in water; sol in propylene glycol; slightly sol in ethanol, 2-propanol.
Melting point: mp >290°
Optical Rotation: [a]D25 -94.1° (c = 0.4 in water)
Therap-Cat: Antiemetic.
Keywords: Serotonin Receptor Antagonist; Antiemetic.