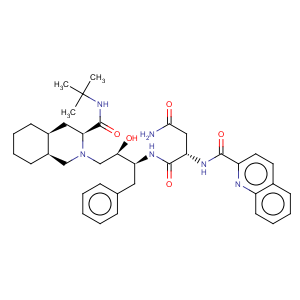
Title: Saquinavir
CAS Registry Number: 127779-20-8
CAS Name: (2
S)-
N1[(1
S,2
R)-3-[(3
S,4a
S,8a
S)-3-[[(1,1-Dimethylethyl)amino]carbonyl]octahydro-2(1
H)-isoquinolinyl]-2-hydroxy-1-(phenylmethyl)propyl]-2-[(2-quinolinylcarbonyl)amino]butanediamide
Synonyms: (
S)-
N-[(a
S)-a-[(1
R)-2-[(3
S,4a
S,8a
S)-3-(
tert-butylcarbamoyl)octahydro-2(1
H)-isoquinolyl]-1-hydroxyethyl]phenethyl]-2-quinaldamido succinamide;
N-tert-butyldecahydro-2-[2(
R)-hydroxy-4-phenyl-3(
S)-[[
N-(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl](4a
S,8a
S)-isoquinoline-3(
S)-carboxamide
Manufacturers' Codes: Ro-31-8959
Molecular Formula: C38H50N6O5
Molecular Weight: 670.84
Percent Composition: C 68.04%, H 7.51%, N 12.53%, O 11.92%
Literature References: Selective HIV protease inhibitor. Prepn: J. A. Martin, S. Redshaw,
EP 432695;
eidem, US 5196438 (1991, 1993 both to Hoffmann-LaRoche); K. E. B. Parkes
et al., J. Org. Chem. 59, 3656 (1994).
In vitro HIV proteinase inhibition: N. A. Roberts
et al., Science 248, 358 (1990). Antiviral properties: J. C. Craig
et al., Antiviral Res. 16, 295 (1991); S. Galpin
et al., Antiviral Chem. Chemother. 5, 43-45 (1994). Clinical evaluation of tolerability and activity: V. S. Kitchen
et al., Lancet 345, 952 (1995). Review of pharmacology and clinical experience: S. Kravcik,
Expert Opin. Pharmacother. 2 303-315 (2001).
Properties: White crystalline solid. [a]D20 -55.9° (c = 0.5 in methanol). Soly (21°): 0.22 g/100 ml water.
Optical Rotation: [a]D20 -55.9° (c = 0.5 in methanol)
Derivative Type: Methanesulfonate salt
CAS Registry Number: 149845-06-7
Synonyms: Saquinavir mesylate
Manufacturers' Codes: Ro-31-8959/003
Trademarks: Fortovase (Roche); Invirase (Roche)
Molecular Formula: C38H50N6O5.CH3SO3H
Molecular Weight: 766.95
Percent Composition: C 61.08%, H 7.10%, N 10.96%, O 16.69%, S 4.18%
Therap-Cat: Antiviral.
Keywords: Antiviral; Peptidomimetics; HIV Protease Inhibitor.