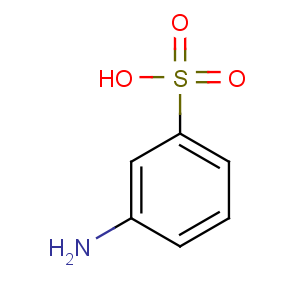
Title: Metanilic Acid
CAS Registry Number: 121-47-1
CAS Name: 3-Aminobenzenesulfonic acid
Synonyms: m-sulfanilic acid; aniline-
m-sulfonic acid
Molecular Formula: C6H7NO3S
Molecular Weight: 173.19
Percent Composition: C 41.61%, H 4.07%, N 8.09%, O 27.71%, S 18.51%
Literature References: Usually obtained by reduction of 3-nitrobenzenesulfonic acid: Fierz-David, Blangey,
Fundamental Processes of Dye Chemistry (Interscience, New York, 1949) pp 120-123; A. I. Vogel,
Practical Organic Chemistry (Longmans, London, 3rd ed., 1959) p 589. Large-scale process:
FIAT Final Rept. 1313 (I), 187-191 (1948). The industrial reduction with iron filings and dil acid gives up to 90% yields, in the lab it seldom exceeds 55%. Better yields with small amounts are claimed for a hydrazine-Raney nickel reduction (about 75%): Gialdi
et al., Farmaco Ed. Sci. 14, 765 (1959) or by using WS2 (about 94%): Ehrmann,
FR 1336648 (1963 to BASF),
C.A. 60, 2846d (1964).
Properties: Anhydrous, orthorhombic needles from water. d 1.69. Very slow crystn yields triclinic prisms of the sesquihydrate. Crystallographic data for both forms: Hall, Maslen,
Acta Crystallogr. 18, 301-306 (1965). Photomicrograph of the sesquihydrate:
Helv. Chim. Acta 12 (1929), facing page 666. Both forms dec on heating without melting. pK (25°) 3.70. Soly of the sesquihydrate in water (16.8°): 2.37% (w/w). The soly of the anhydr form in water is given as 0.79% (w/w) at 0° and as 6.50% (w/w) at 85°. Sparingly sol in methanol, ethanol.
pKa: pK (25°) 3.70
Density: d 1.69
Derivative Type: Sodium salt
Molecular Formula: H2NC6H4SO3Na
Molecular Weight: 195.17
Percent Composition: H 3.10%, N 7.18%, C 36.92%, S 16.43%, O 24.59%, Na 11.78%
Properties: Minute crystals from water, dec 302-304°.
Use: The sodium salt in the manuf of azo dyes. In the synthesis of certain sulfa drugs.