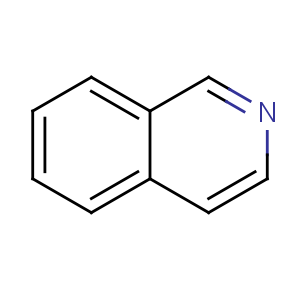
Title: Isoquinoline
CAS Registry Number: 119-65-3
Synonyms: 2-Benzazine; benzo[
c]pyridine
Molecular Formula: C9H7N
Molecular Weight: 129.16
Percent Composition: C 83.69%, H 5.46%, N 10.84%
Literature References: Occurs in coal tar: Hoogewerff, van Dorp,
Rec. Trav. Chim. 4, 125, 285 (1885); Weissgerber,
Ber. 47, 3175 (1914); Harris, Pope,
J. Chem. Soc. 121, 1029 (1922). Modern isoln processes: H. J. V. Winkler,
Der Steinkohlenteer und seine Aufarbeitung (Essen, 1951). Isoln by a freeze-out method: Kjellman,
US 2483420 (1946 to Koppers). Purification: Freiser, Glowacki,
J. Am. Chem. Soc. 71, 514 (1949). Synthesis: D. L. Boger
et al., Tetrahedron 37, 3977 (1981). Toxicity data: Smyth
et al., Arch. Ind. Hyg. Occup. Med. 4, 119 (1951). Comprehensive survey of isoquinoline alkaloids: Gensler,
Heterocyclic Compounds vol. 4, R. C. Elderfield, Ed. (Wiley, New York, 1952) pp 344-490; T. Kametani,
Total Synthesis of Natural Products vol. 3, J. ApSimon, Ed. (Wiley, New York, 1977) pp 1-272.
Properties: Liquid. Pungent odor resembling that of a mixture of anise oil and benzaldehyde. Hygroscopic platelets when solid. d430 1.09101; d480 1.05143. mp 26.48°.
nD30 1.62078. Viscosity (cP): 3.2528 (30°); 1.0230 (100°); 0.4223 (200°). bp743 242.2°; bp760 243.25°. Dipole moment: 2.49. More basic than quinoline, pKa (25°): 8.60. Almost insol in water. Miscible with many organic solvents. Sol in dil acids. LD50 orally in rats: 360 mg/kg (Smyth).
Melting point: mp 26.48°
Boiling point: bp743 242.2°; bp760 243.25°
pKa: pKa (25°): 8.60
Index of refraction: nD30 1.62078
Density: d430 1.09101; d480 1.05143
Toxicity data: LD50 orally in rats: 360 mg/kg (Smyth)
Derivative Type: Sulfate
Molecular Formula: C9H7N.H2SO4
Molecular Weight: 227.24
Percent Composition: C 47.57%, H 3.99%, N 6.16%, S 14.11%, O 28.16%
Properties: Crystals, mp 209-209.5°. Sol in water.
Melting point: mp 209-209.5°
Use: Synthesis of dyes, insecticides, antimalarials, rubber accelerators.