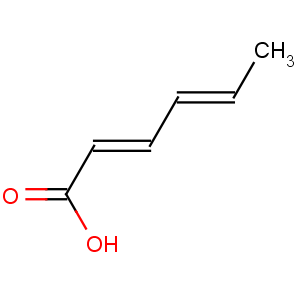
Title: Sorbic Acid
CAS Registry Number: 110-44-1
CAS Name: (2
E,4
E)-2,4-Hexadienoic acid
Synonyms: 2-propenylacrylic acid
Molecular Formula: C6H8O2
Molecular Weight: 112.13
Percent Composition: C 64.27%, H 7.19%, O 28.54%
Line Formula: CH3CH=CHCH=CHCOOH
Literature References: May be obtained from berries of the mountain ash,
Sorbus aucuparia L.,
Rosaceae where it occurs as the lactone, called parasorbic acid: Hofmann,
Ann. 110, 129 (1859). Synthesis by condensing crotonaldehyde and malonic acid in pyridine soln: Doebner,
Ber. 33, 2140 (1900); Allen, Van Allan,
Org. Synth. coll. vol. III, 783 (1955); by condensing crotonaldehyde and ketene in the presence of boron trifluoride: Hagemeyer, Jr.,
Ind. Eng. Chem. 41, 768 (1949). Prepn from 1,1,3,5-tetraalkoxyhexane: Parker, MacLean,
US 2921090 (1960 to Celanese). Additional syntheses: Fernholz, Mundlos,
DE 1049852;
US 3021365 (1959, 1962 both to Hoechst). Prepn of sodium salt: Horn, Fernholz,
DE 1045390 (1958 to Hoechst); of calcium salt: C. M. Gooding,
US 3139378 (1964 to Corn Products Co.). Purification: Fernholz,
DE 1044803 (1958 to Hoechst). The
trans,trans-isomer is usually obtained and is the commercial product. Toxicity study: Smyth, Carpenter,
J. Ind. Hyg. Toxicol. 30, 63 (1948).
Properties: Needles from water, mp 134.5°. Should be stored at temps below 40°. bp 228° (dec). Vapor pressure at 20° <0.01 mm, at 143° 50 mm. Flash pt 260°F (127°C). pK (25°) = 4.76. Soly in water at 30° 0.25%, at 100° 3.8%, in propylene glycol at 20° 5.5%, in abs ethanol or methanol 12.90%, in 20% ethanol 0.29%, in glacial acetic acid 11.5%, in acetone 9.2%, in benzene 2.3%, in carbon tetrachloride 1.3%, in cyclohexane 0.28%, in dioxane 11.0%, in glycerol 0.31%, in isopropanol 8.4%, in isopropyl ether 2.7%, in methyl acetate 6.1%, in toluene 1.9%. LD50 orally in rats: 7.36 g/kg (Smyth, Carpenter).
Melting point: mp 134.5°
Boiling point: bp 228° (dec)
Flash point: Flash pt 260°F (127°C)
pKa: pK (25°) = 4.76
Toxicity data: LD50 orally in rats: 7.36 g/kg (Smyth, Carpenter)
Use: Mold and yeast inhibitor. Fungistatic agent for foods, especially cheeses. To improve the characteristics of drying oils. In alkyd type coatings to improve gloss. To improve milling characteristics of cold rubber.
See also Potassium Sorbate.