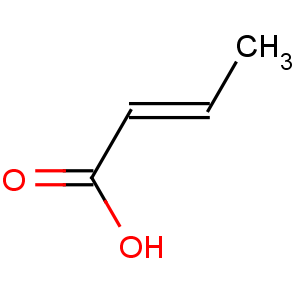
Title: Crotonic Acid
CAS Registry Number: 107-93-7
CAS Name: (2
E)-2-Butenoic acid
Synonyms: b-methylacrylic acid; a-crotonic acid; solid crotonic acid;
trans-crotonic acid
Molecular Formula: C4H6O2
Molecular Weight: 86.09
Percent Composition: C 55.81%, H 7.02%, O 37.17%
Literature References: Has been found in clay soil in Texas; formed during the dry distillation of wood. Obtained on a commercial scale exclusively by oxygen- or air-oxidation of crotonaldehyde: Kennedy,
US 2413235 (1946 to Shawinigan Chem.); Leupold, PB report 70249 (1942); Matthews, B.I.O.S. report 758 (1946). Laboratory procedure using alkaline silver oxide: Young,
J. Am. Chem. Soc. 54, 2498 (1932); from acetaldehyde and malonic acid in pyridine: v. Auwers,
Ann. 432, 46 (1923); Backer, Bloemen,
Rec. Trav. Chim. 45, 102 (1926); Florence,
Bull. Soc. Chim. Fr. [4]
41, 440 (1927); Letch, Linstead,
J. Chem. Soc. 1932, 454. Toxicity study: Smyth, Carpenter,
J. Ind. Hyg. Toxicol. 26, 269 (1944).
Review: W. Blau
et al., in
Ullmann's Encyclopedia of Industrial Chemistry vol. A8 (VCH, Weinheim, 5th ed., 1987) pp 83-89.
Properties: Monoclinic needles, prisms from water or ligroin. Corrosive and combustible solid. d415 1.018; d480 0.964. mp 71.6°. bp10 80.0°; bp20 93.0°; bp40 107.8°; bp60 116.7°; bp100 128.0°; bp200 146.0°; bp400 165.5°; bp760 185.0°.
nD80 1.4228. pKa (25°) 4.817. Heat of combustion: 2.00 MJ/mol. Heat of fusion: 150.9 J/g. Soly in water (g/l) at 0°: 41.5; at 10°: 54.6; at 20°: 76.1; at 25°: 94; at 30°: 122; at 40°: 656. Soly in ethanol at 25°: 52.5% w/w; acetone: 53.0% w/w; toluene: 37.5% w/w. LD50 orally in rats: 1.0 g/kg (Smyth, Carpenter).
Melting point: mp 71.6°
Boiling point: bp10 80.0°; bp20 93.0°; bp40 107.8°; bp60 116.7°; bp100 128.0°; bp200 146.0°; bp400 165.5°; bp760 185.0°
pKa: pKa (25°) 4.817
Index of refraction: nD80 1.4228
Density: d415 1.018; d480 0.964
Toxicity data: LD50 orally in rats: 1.0 g/kg (Smyth, Carpenter)
Derivative Type: Methyl ester
Molecular Formula: C5H8O2
Molecular Weight: 100.12
Percent Composition: C 59.98%, H 8.05%, O 31.96%
Properties: Liq, bp 121°; d420 0.9444;
nD20 1.4242.
Boiling point: bp 121°
Index of refraction: nD20 1.4242
Density: d420 0.9444
Derivative Type: Ethyl ester
Molecular Formula: C6H10O2
Molecular Weight: 114.14
Percent Composition: C 63.14%, H 8.83%, O 28.03%
Properties: Liq, bp 138°; d420 0.9175;
nD20 1.4245.
Boiling point: bp 138°
Index of refraction: nD20 1.4245
Density: d420 0.9175
Derivative Type: Vinyl ester
Molecular Formula: C6H8O2
Molecular Weight: 112.13
Percent Composition: C 64.27%, H 7.19%, O 28.54%
Properties: Liq, bp 133°; d420 0.9410;
nD20 1.450.
Boiling point: bp 133°
Index of refraction: nD20 1.450
Density: d420 0.9410
Use: In the manuf of copolymers with vinyl acetate used in lacquers and paper sizing; in the manuf of softening agents for synthetic rubber. In medicinal chemistry, e.g., in the manuf of DL-threonine, vitamin A.