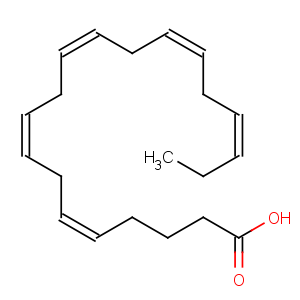
Title: Eicosapentaenoic Acid
CAS Registry Number: 10417-94-4
CAS Name: (5
Z,8
Z,11
Z,14
Z,17
Z)-5,8,11,14,17-Eicosapentaenoic acid
Synonyms: (
all-Z)-5,8,11,14,17-eicosapentaenoic acid;
all-cis-fatty acid 20:5 omega-3; EPA; icosapent
Molecular Formula: C20H30O2
Molecular Weight: 302.45
Percent Composition: C 79.42%, H 10.00%, O 10.58%
Literature References: Important polyunsaturated fatty acid of the marine food chain that serves as a precursor for the prostaglandin-3 and thromboxane-3 families. It differs from arachidonic acid,
q.v. (the eicosatetraenoic acid that is a precursor for the prostaglandin and thromboxane-2 families) by the extra double bond between the third and fourth carbons from the "methyl end" of the molecule. Isoln from cod liver oil: E. Klenk, D. Eberhagen,
Z. Physiol. Chem. 307, 42 (1957). Enzymatic conversion to prostaglandin E3: S. Bergstr?m
et al., J. Biol. Chem. 239, PC 4006 (1964). Effects on role of platelets in thrombosis: K. C. Srivastava
et al., Biochem. Exp. Biol. 16, 317 (1980). Effects on prostacyclin-like material in human umbilical vasculature: J. Dyerberg, K. A. Jorgensen,
Artery 8, 12 (1980). A possible relationship between diets rich in EPA in marine oils and low rates of ischemic heart disease has been proposed: H. O. Bang
et al., Am. J. Clin. Nutr. 33, 2657 (1980); J. Dyerberg,
Philos. Trans. R. Soc. London Ser. B 294, 373 (1981). Clinical evaluation of lipid lowering effect: Y. Nagakawa
et al., Atherosclerosis 47, 71 (1983); of use in rheumatoid arthritis: J. M. Kremer
et al., Ann. Intern. Med. 106, 497 (1987); of use in Raynaud's phenomenon: R. A. DiGiacomo
et al., Am. J. Med. 86, 158 (1989).
Properties: Colorless oil.
nD20 1.49865.
Index of refraction: nD20 1.49865
Derivative Type: Ethyl ester
CAS Registry Number: 86227-47-6
Trademarks: Epadel (Mochida)
Molecular Formula: C22H34O2
Molecular Weight: 330.50
Percent Composition: C 79.95%, H 10.37%, O 9.68%
Therap-Cat: Antilipemic.
Keywords: Antilipemic.