References of OctopamineOctopamine 4-(2-Amino-1-hydroxyethyl)phenolOctopamineOctopamineOctopamineOctopamine 4-(2-Amino-1-hydroxyethyl)phenol1-(4-Hydroxyphenyl)-2-aminoethanolOctopamineOctopamine 4-(2-Amino-1-hydroxyethyl)phenolOctopamineOctopamineOctopamineOctopamine 4-(2-Amino-1-hydroxyethyl)phenol1-(4-Hydroxyphenyl)-2-aminoethanolOctopamineOctopamine 4-(2-Amino-1-hydroxyethyl)phenolOctopamineOctopamineOctopamineOctopamine 4-(2-Amino-1-hydroxyethyl)phenol1-(4-Hydroxyphenyl)-2-aminoethanolOctopamineOctopamine 4-(2-Amino-1-hydroxyethyl)phenolOctopamineOctopamineOctopamineOctopamine 4-(2-Amino-1-hydroxyethyl)phenol1-(4-Hydroxyphenyl)-2-aminoethanol
Title: Octopamine
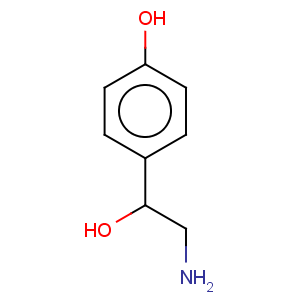
CAS Registry Number: 104-14-3
CAS Name: a-(Aminomethyl)-4-hydroxybenzenemethanol
Synonyms: a-(aminomethyl)-
p-hydroxybenzyl alcohol; 1-(
p-hydroxyphenyl)-2-aminoethanol; norsympatol; norsynephrine;
p-hydroxyphenylethanolamine
Manufacturers' Codes: WV-569
Molecular Formula: C8H11NO2
Molecular Weight: 153.18
Percent Composition: C 62.73%, H 7.24%, N 9.14%, O 20.89%
Literature References: A biogenic amine that is the phenol analog of noradrenaline (norepinephrine,
q.v.). It is a neurosecretory product found in several vertebrates and invertebrates. Formed by b-hydroxylation of tyramine by the enzyme dopamine b-hydroxylase: Pisano
et al., Biochim. Biophys. Acta 43, 566 (1960). Identification: Erspamer,
Nature 169, 375 (1952). Found in the salivary glands of
Octopus vulgaris, O. macropus, and of
Eledone moschata: idem, Arzneim.-Forsch. 2, 253 (1952); in mammalian nerves: Molinoff, Axelrod,
Science 164, 428 (1969); in cockroach nervous system: Nathanson, Greengard,
ibid. 180, 308 (1973). Prepd synthetically: Asscher,
US 2585988 (1952). The natural D(-) form is 3 times more potent than the L(+) form in producing cardiovascular adrenergic responses in anesthetized dogs and cats: Korol, Soffer,
Pharmacologist 5, 247 (1963). Prepn of D- and L-forms: Kappe, Armstrong,
J. Med. Chem. 7, 569 (1964). In invertebrate nervous systems octopamine may function as a neurotransmitter: Saavedra
et al., Science 185, 364 (1974). Effects on neuromuscular transmission in crustacean muscle: C. A. Breen, H. L. Atwood,
Nature 303, 716 (1983).
Derivative Type: D(-)-Form
CAS Registry Number: 876-04-0
Properties: Crystals from hot water which change at about 160° to a compd which melts above 250° (dec). [a]D25 -56.0° (0.1
N HCl); -37.4° (H2O).
Optical Rotation: [a]D25 -56.0° (0.1
N HCl); -37.4° (H2O)
Derivative Type: DL-Form hydrochloride
CAS Registry Number: 770-05-8
Trademarks: Epirenor (Byk Gulden); Norden; Norfen (Morishita); Norphen (ampules) (Byk Gulden)
Molecular Formula: C8H11NO2.HCl
Molecular Weight: 189.64
Percent Composition: C 50.67%, H 6.38%, N 7.39%, O 16.87%, Cl 18.69%
Properties: Crystals, dec 170°. Freely sol in water.
Therap-Cat: Adrenergic.
Keywords: a-Adrenergic Agonist.