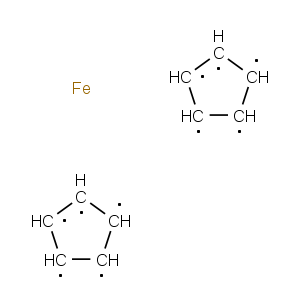
Title: Ferrocene
CAS Registry Number: 102-54-5
Synonyms: Dicyclopentadienyliron; biscyclopentadienyliron
Molecular Formula: C10H10Fe
Molecular Weight: 186.03
Percent Composition: C 64.56%, H 5.42%, Fe 30.02%
Literature References: Prepn: Kealy, Pauson,
Nature 168, 1039 (1951); Pauson,
US 2680756 (1954 to Du Pont); Miller
et al., J. Chem. Soc. 1952, 632; Anzilotti, Weinmayr,
US 2791597 (1957 to Du Pont). Other prepn: Pruett, Morehouse in
Adv. Chem. Ser. 23, entitled "Metal-Organic Compounds," M. Sittig, Ed. (ACS, Washington DC, 1959) pp 368-371; Wilkinson,
Org. Synth. coll. vol. IV, 473 (1963); Cordes,
FR 1341880 (1963 to BASF),
C.A. 60, 6873a (1964). Structure studies: Wilkinson
et al., J. Am. Chem. Soc. 74, 2125 (1952); Seibold, Sutton,
J. Chem. Phys. 23, 1967 (1955). Synthesis of a helical ferrocene: T. J. Katz, J. Pesti,
J. Am. Chem. Soc. 104, 346 (1982).
Reviews: Rausch
et al., J. Chem. Educ. 34, 268-272 (1957); M. Rosenblum,
Chemistry of the Iron Group Metallocenes (John Wiley, New York, 1965) 241 pp; Bruce,
Organomet. Chem. Rev. Sect. B 10, 75-122 (1972); B. W. Rockett, G. Marr,
J. Organomet. Chem. 211, 215-278 (1981).
Properties: Orange needles from methanol or ethanol; odor of camphor. mp 173-174°. Sublimes above 100°. Volatile in steam. Practically insol in water, 10% NaOH, and concd boiling HCl. Sol in alcohol, ether, benzene. Dissolves in dil nitric and concd sulfuric acids forming a deep red soln with blue fluorescence. The molecule is diamagnetic and the dipole moment is effectively zero.
Melting point: mp 173-174°
CAUTION: Potential symptoms of overexposure are irritation of eyes, skin and respiratory system.
See NIOSH Pocket Guide to Chemical Hazards (DHHS/NIOSH 97-140, 1997) p 104.
Use: Antiknock additive for gasoline; catalyst.