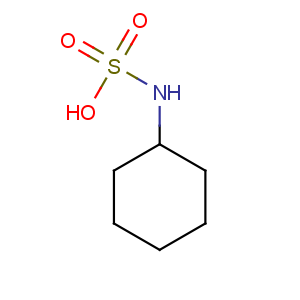
Title: Cyclamic Acid
CAS Registry Number: 100-88-9
CAS Name: Cyclohexylsulfamic acid
Synonyms: cyclohexanesulfamic acid
Trademarks: Hexamic Acid (Abbott)
Molecular Formula: C6H13NO3S
Molecular Weight: 179.24
Percent Composition: C 40.21%, H 7.31%, N 7.81%, O 26.78%, S 17.89%
Literature References: Prepn: Audrieth, Sveda,
US 2275125 (1942 to du Pont);
J. Org. Chem. 9, 89 (1944); Thompson,
US 2800501 (1957 to du Pont); Shah, Bernsen,
US 3361799 (1968 to Abbott). Prepn of the Na salt: Robinson,
US 2383617 (1945 to du Pont). Other prepns and metabolism:
See Calcium Cyclamate. Sweetness of the sodium salt discovered by Michael Sveda at the University of Illinois in 1937. Toxicity: Taylor
et al., Food Cosmet. Toxicol. 6, 313 (1968). Review of long-term toxicity and carcinogenicity of sodium cyclamate in mice: Brantom
et al., Food Cosmet. Toxicol. 11, 735 (1973).
Properties: Sweet-sour crystals. mp 169-170°. Fairly strong acid. Very sparingly soluble in water. Slowly hydrolyzed by hot water.
Melting point: mp 169-170°
Derivative Type: Sodium salt
Synonyms: Sodium cyclohexylsulfamate; cyclamate sodium; sodium cyclamate
Trademarks: Assugrin; Sucaryl Sodium; Sucrosa
Molecular Formula: C6H12NNaO3S
Molecular Weight: 201.22
Percent Composition: C 35.81%, H 6.01%, N 6.96%, Na 11.43%, O 23.85%, S 15.94%
Properties: Crystals. Pleasantly sweet to the taste. Freely sol in water. About 30 times as sweet as refined cane sugar. Sweetness is still easily perceptible at a dilution of 1:10,000 (sugar 1:140; saccharin 1:50,000). pH of 10% aq soln between 5.5 and 7.5. Practically insol in alcohol, ether, benzene, chloroform. LD50 orally in mice, rats: 17.0, 15.25 g/kg (Taylor).
Toxicity data: LD50 orally in mice, rats: 17.0, 15.25 g/kg (Taylor)
NOTE: Consult latest Government regulations on use in food.
Use: Non-nutritive sweetener.