Quick Detail:
Product name
Dutasteride Factory Supplying
Other name
DUTASTERIDE; Duagen; Avodart
CAS register number
164656-23-9
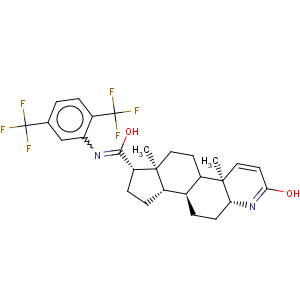
Molecular formula
C27H30F6N2O2
Molecular weight
528.53
Molecular structure
Avodart / Dutasteride Dentist Anesthetic Anodyne 164656-23-9 Duagen / Dutasteride
Melting point
242°C~250°C
Assay
99%
Product description:
Dutasteride (trademark name Avodart) is a dual 5-α reductase inhibitor that inhibits conversion of testosterone to dihydrotestosterone (DHT). Used for benign prostatic hyperplasia; however, it increases the risk of erectile dysfunction and decreased sexual desire.
Dutasteride useful for the symptoms of benign prostatic hyperplasia (BPH); colloquially known as an "enlarged prostate".
In those who are being regularly screened 5-alpha-reductase inhibitor (finasteride and dutasteride) reduce the overall risk of being diagnosed with prostate cancer however there is insufficient data to determine if they have an effect on the risk of death and may increase the chance of more serious cases.
This class of medications increases rates of erectile dysfunction (with between 5% and 9% developing problems after starting their use). This is linked to lower quality of life and can cause stress in relationships. There is also an association with lowered sexual desire. It has been reported that these adverse sexual side effects may persist even after discontinuation of the drug.
The FDA has added a warning to dutasteride about an increased risk of high-grade prostate cancer. While the potential for positive, negative or neutral changes to the potential risk of developing prostate cancer with dutasteride has not been established, evidence has suggested it may temporarily reduce the growth and prevalence of benign prostate tumors, but could also mask the early detection of prostate cancer. The primary area for concern is for patients who may develop prostate cancer whilst taking dutasteride for benign prostatic hyperplasia, which in turn could delay diagnosis and early treatment of the prostate cancer, thereby potentially increasing the risk of these patients developing high-grade prostate cancer.
COA and HPLC:
COA:
Product name
Dutasteride
Appearance
White crystalline powder
Assay(*)
98~101.0%
99.4%
Identification
By UV, To match with working standard
Complies
Related Substances
Not more than the reference solution (0.05%)
Complies
Heavy metals
≤10ppm
9ppm
Loss on drying
<0.5%
0.2%
Resiue on ignition
≤0.1%
0.05%
Melting
242°C~250°C
245°C
For detail, please contact:
Dawn (miss)
Email: cherry@ycphar.com
Skype: dong.wen6
Our website: http://steroid.en.made-in-china.com
Permanent link: http://www.vvchem.com/sell/cas:164656-23-9,3288332.html