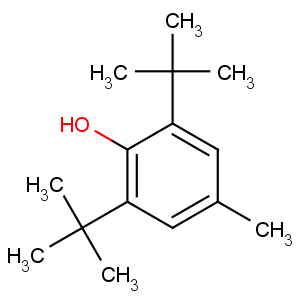
Product Name: 2,6-Di-tert-butyl-4-methylphenol
Synonyms: butyleret hydroxytoluen(= 2,6-di-tert-butyl-p-cresol);2,6-Di-tert-butyl-4-methylphenol, 99.8%;2,6-Di-(tert-butyl)-4-methylphynol-D21;3,5-di-tert-4butylhydroxytoluene (bht);BHT (BAGS);BHT FCC/NF;BHT,GRANULAR,FCC;BHT,GRANULAR,TECHNICAL
CAS: 128-37-0
Appearance: White Crystalline Powder
Molecular Formula: C15H24O
Molecular Weight: 220.35
EINECS: 204-881-4
Product Categories: Industrial/Fine Chemicals;Aromatic Hydrocarbons (substituted) & Derivatives;Antioxidant;Biochemistry;Aromatics;BHT;antioxidants;Food additive
Mol File: 128-37-0.mol
Melting Point: 69-73?°C(lit.)
Boiling Point: 265?°C(lit.)
Density? 1.048
vapor density? 7.6 (vs air)
vapor pressure? <0.01 mm Hg ( 20 °C)
FEMA? 2184
refractive index? 1.4859
Flame Point: 127 °C
storage temp.? 0-6°C
solubility? methanol: 0.1?g/mL, clear, colorless
Water Solubility? insoluble
Merck? 14,1548
BRN? 1911640
Stability: Stable, but light-sensitive. Incompatible with acid chlorides, acid anhydrides, brass, copper, copper alloys, steel, bases, oxidizing agents. Combustible.
CAS DataBase Reference 128-37-0(CAS DataBase Reference)
NIST Chemistry Reference Butylated hydroxytoluene(128-37-0)
EPA Substance Registry System Phenol, 2,6-bis(1,1-dimethylethyl)- 4-methyl-(128-37-0)
HS Code? 29071900
Hazardous Substances Data 128-37-0(Hazardous Substances Data)
Usage Antioxidant 264 as general antioxidants is used widely in polymer materials, petroleum products and food processing industries. Antioxidant 264 is commonly used rubber antioxidant, heat, oxygen aging have some protective effect, but also can inhibit copper harm. This product does not change color, not pollution. Antioxidants 264 high solubility in oil, no precipitation, less volatile, non-toxic and non-corrosive.
General Description White crystalline solid.
Air & Water Reactions Insoluble in water.
Reactivity Profile Phenols, such as 2,6-Di-tert-butyl-4-methylphenol, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid. Nitrated phenols often explode when heated. Many of them form metal salts that tend toward detonation by rather mild shock. May react with oxidizing materials.
Fire Hazard 2,6-Di-tert-butyl-4-methylphenol is combustible.
Permanent link: http://www.vvchem.com/sell/cas:128-37-0,3317324.html