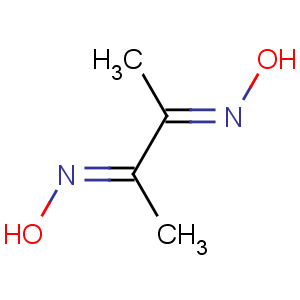
Title: Dimethylglyoxime
CAS Registry Number: 95-45-4
CAS Name: 2,3-Butanedionedioxime
Synonyms: diacetyldioxime; Chugaev's reagent
Molecular Formula: C4H8N2O2
Molecular Weight: 116.12
Percent Composition: C 41.37%, H 6.94%, N 24.12%, O 27.56%
Literature References: Prepn: Semon, Damerell,
Org. Synth. coll. vol. II, 204 (1943). Manuf: Kamlet,
US 2732404 (1956 to National Distillers Product Corp.). Crystal structure:
Anal. Chem. 21, 1428 (1949); Merritt, Lanterman,
Acta Crystallogr. 5, 811 (1952).
Properties: Triclinic crystals from alc + water, mp 238-240°, also reported as melting 242° and 246°. Practically insol in water. Sol in alc, ether, pyridine, acetone.
Melting point: mp 238-240°, also reported as melting 242° and 246°
Use: Detection and determination of Ni and its separation from Co and many other metals. Forms a scarlet red ppt with Ni even in dil solns. Separation of Pd from Sn, Au, Rh, and Ir, also to detect Bi with which it forms a bright yellow color and ppt.