References of (2E,4E)-5-(1,3-benzodioxol-5-yl)-1-piperidin-1-ylpenta-2,4-dien-1-one
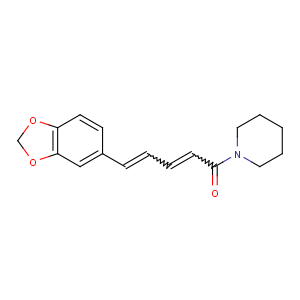
Title: Piperine
CAS Registry Number: 94-62-2
CAS Name: 1-[(2
E,4
E)-5-(1,3-Benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]piperidine
Synonyms: (
E,E)-1-piperoylpiperidine
Molecular Formula: C17H19NO3
Molecular Weight: 285.34
Percent Composition: C 71.56%, H 6.71%, N 4.91%, O 16.82%
Literature References: Isolated from black pepper (
Piper nigrum L.); also in
P. longum L.,
P. retrofractum Vahl. (
P. officinarum C.D.C.), and
P. clusii C.D.C.; in root bark of
Piper geniculatum Sw.,
Piperaceae. Extraction procedure: Cazeneuve, Caillot,
Bull. Soc. Chim. [2]
27, 291 (1877). Synthesis: Rugheimer,
Ber. 15, 1390 (1882); Newman,
Chem. Prod. 16, 379 (1953); Normant, Feugeas,
Compt. Rend. 258, 2846 (1964). Spectroscopic structural elucidation and preparative separation of piperine and its stereoisomers isopiperine, isochavicine and chavicine,
q.v.: R. De Cleyn, M. Verzele,
Bull. Soc. Chim. Belg. 84, 435 (1975). Synthesis of isomers: R. Grewe
et al., Ber. 103, 3752 (1970); of piperine and isochavicine: S. Tsuboi
et al., Tetrahedron Lett. 1979, 1043. Stereoselective synthesis of piperine: R. A. Olsen, G. O. Spessard,
J. Agric. Food Chem. 29, 942 (1981). More toxic to houseflies than pyrethrum: Harvill
et al., Contrib. Boyce Thompson Inst. 13, 87 (1943).
Properties: Monoclinic prisms from alcohol, mp 130°. Tasteless at first, but burning aftertaste. Neutral to litmus. pK (18°): 12.22. Almost insol in water (40 mg/liter at 18°), in petr ether. One gram dissolves in 15 ml alcohol, 1.7 ml chloroform, 36 ml ether. Sol in benzene, acetic acid.
Melting point: mp 130°
pKa: pK (18°): 12.22
Derivative Type: (E,Z)-Form
Synonyms: Isochavicine
Properties: Crystals from chloroform + hexane, mp 89° (Grewe), 103° (De Cleyn). uv max (methanol): 333 nm (e 16300).
Melting point: mp 89° (Grewe), 103° (De Cleyn)
Absorption maximum: uv max (methanol): 333 nm (e 16300)
Derivative Type: (Z,E)-Form
Synonyms: Isopiperine
Properties: Crystals from chloroform + hexane, mp 110° (Grewe), 86° (De Cleyn). uv max (methanol): 332 nm (e 21800).
Melting point: mp 110° (Grewe), 86° (De Cleyn)
Absorption maximum: uv max (methanol): 332 nm (e 21800)
Use: To impart pungent taste to brandy. As insecticide.