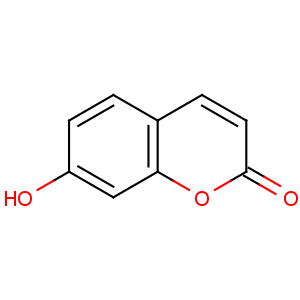
Title: Umbelliferone
CAS Registry Number: 93-35-6
CAS Name: 7-Hydroxy-2
H-1-benzopyran-2-one
Synonyms: 7-hydroxycoumarin; hydrangin; skimmetin
Molecular Formula: C9H6O3
Molecular Weight: 162.14
Percent Composition: C 66.67%, H 3.73%, O 29.60%
Literature References: The aglucon of skimmin. Present in many plants. Obtained by distillation of resins from umbelliferae: Zwenger,
Ann. 115, 1, 15 (1860). Prepn: Bert,
Compt. Rend. 214, 230 (1942); Austerweil,
ibid. 248, 1810 (1959); Dressler, Reabe,
US 3503996 (1970 to Koppers). Main product of metabolism of coumarin in man: Schilling
et al., Nature 221, 664 (1969). Metabolism studies of umbelliferone: Indahl, Scheline,
Xenobiotica 1, 13 (1971). Use in brain intracellular pH measurements: T. M. Sundt, R.E. Anderson,
Brain Res. 186, 355 (1980);
eidem, J. Neurophysiol. 44, 60 (1980). Use in fluorescent immunoassays: S. G. Thompson, J. F. Burd,
Antimicrob. Agents Chemother. 18, 264 (1980); T. M. Li
et al., Anal. Biochem. 118, 102 (1981).
Properties: Needles from water, mp 225-228°. Develops odor of coumarin on heating. Sublimes. Absorption spectrum: Sen, Bagchi,
J. Org. Chem. 24, 316 (1959). One gram dissolves in ~100 ml boiling water; freely sol in alcohol, chloroform, acetic acid; sol in dil alkalies; sparingly sol in ether. Solns show blue fluorescence.
Melting point: mp 225-228°
Use: In sunscreen lotions and creams; as intracellular and pH sensitive fluorescent indicator and blood-brain barrier probe.