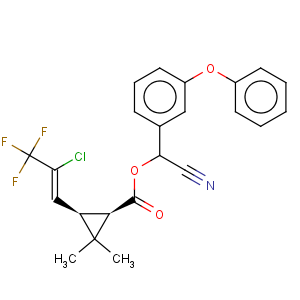
Title: l-Cyhalothrin
CAS Registry Number: 91465-08-6
CAS Name: (1
S,3
S)-
rel-3-[(1
Z)-2-Chloro-3,3,3-trifluoro-1-propenyl]-2,2-dimethylcyclopropanecarboxylic acid (
R)-cyano(3-phenoxyphenyl)methyl ester
Synonyms: (±)-a-cyano-3-phenoxybenzyl 3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropane carboxylate
Manufacturers' Codes: PP-321
Trademarks: Karate (Syngenta); Warrior (Syngenta)
Molecular Formula: C23H19ClF3NO3
Molecular Weight: 449.85
Percent Composition: C 61.41%, H 4.26%, Cl 7.88%, F 12.67%, N 3.11%, O 10.67%
Literature References: Synthetic pyrethroid; 1:1 mixture of the
Z-(1
R,3
R)
S-ester isomers and
Z-(1
S,3
S)
R-ester isomers of cyhalothrin,
q.v. Prepn: M. J. Robson, J. Crosby,
EP 106469; J. Crosby,
US 4510098 (1984, 1985 both to ICI); M. J. Robson
et al., Brighton Crop Prot. Conf. - Pests Dis. 1984, 853. Description of chemical properties and biological activities: A. R. Jutsum
et al., ibid. 421. Field trials for mosquito control: A. A. Weathersbee
et al., J. Am. Mosq. Control Assoc. 7, 238 (1991); for sandfly control: D. A. Elnaiem
et al., Med. Vet. Entomol. 13, 310 (1999). Effect on beneficial insects: J. S. White
et al., Brighton Crop Prot. Conf. - Pests Dis. 1990, 969. Dissipation on soil: B. D. Hill, D. J. Inaba,
J. Agric. Food Chem. 39, 2282 (1991). Determination in natural waters: S. T. Hadfield
et al., Pestic. Sci. 34, 207 (1992). Review of early field trials on horticultural crops: D. Wilson, J. Trevenna,
Brighton Crop Prot. Conf. - Pests Dis. 1986, 323.
Properties: White odorless solid, mp 49.2°. Vapor pressure at 20°: 2 ′ 10-10 kPa. Soly (mg/l): purified water 5 ′ 10-3; buffered water 4 ′ 10-3. Sol in common range of solvents at 21°C. LD50 (technical grade) in male, female rats (mg/kg): 79, 56 orally; 632, 696 dermally (Jutsum).
Melting point: mp 49.2°
Toxicity data: LD50 (technical grade) in male, female rats (mg/kg): 79, 56 orally; 632, 696 dermally (Jutsum)
Use: Insecticide.