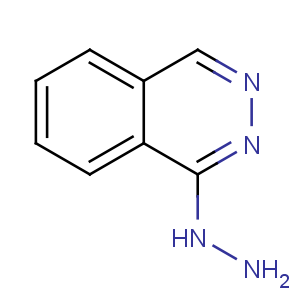
Title: Hydralazine
CAS Registry Number: 86-54-4
CAS Name: 1(2
H)-Phthalazinone hydrazone
Synonyms: 1-hydrazinophthalazine
Manufacturers' Codes: Ciba 5968; Pr?parat 5968; C-5968
Molecular Formula: C8H8N4
Molecular Weight: 160.18
Percent Composition: C 59.99%, H 5.03%, N 34.98%
Literature References: Prepd by the action of hydrazine hydrate on 1-chloro- or 1-phenoxyphthalazine: Hartmann, Druey,
US 2484029 (1949 to Ciba); Druey, Ringier,
Helv. Chim. Acta 34, 204 (1951). Metabolism: Z. H. Israile, P. G. Dayton,
Drug Metab. Rev. 6, 283 (1977); K. Schmid
et al., Arzneim.-Forsch. 31, 1143 (1981). Pharmacology: J. L. Cangiano
et al., J. Lab. Clin. Med. 92, 516 (1978). Clinical paper: R. F. Albrecht
et al., Int. Anesthesiol. Clin. 16, 299 (1978). Acute toxicity: L. Dorigotti
et al., Pharmacol. Res. Commun. 8, 295 (1976). Comprehensive description: C. E. Orzech
et al., Anal. Profiles Drug Subs. 8, 283-314 (1979).
Properties: Yellow needles from methanol, mp 172-173° (rapid heating). One gram dissolves in 3 ml 2
N acetic acid, in 12 ml warm methanol. Forms a red compd (phthalazinylhydrazone) with acetone at 60° in presence of 2
N acetic acid. LD50 in mice, rats (mg/kg): 122, 90 orally; 101, 40 i.p. (Dorigotti).
Melting point: mp 172-173° (rapid heating)
Toxicity data: LD50 in mice, rats (mg/kg): 122, 90 orally; 101, 40 i.p. (Dorigotti)
Derivative Type: Hydrochloride
CAS Registry Number: 304-20-1
Trademarks: Alphapress (Alphapharm); Apresoline (Novartis)
Molecular Formula: C8H8N4.HCl
Molecular Weight: 196.64
Percent Composition: C 48.86%, H 4.61%, N 28.49%, Cl 18.03%
Properties: Yellow crystals, dec 273°. Soly in water (g/100 ml) at 15°: 3.01; at 25°: 4.42; in 95% ethanol: 0.2 g/100 ml. Very slightly sol in ether. pH of a 2% aq soln 3.5 to 4.5. uv max (0.001% aq soln): 211, 240, 260, 304, 315 nm. Aq solns containing 20 mg/ml may be preserved with 0.5% chlorobutanol.
Absorption maximum: uv max (0.001% aq soln): 211, 240, 260, 304, 315 nm
Therap-Cat: Antihypertensive.
Keywords: Antihypertensive; Hydrazines/Phthalazines.