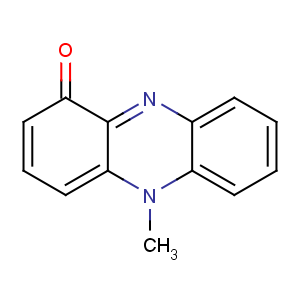
Title: Pyocyanine
CAS Registry Number: 85-66-5
CAS Name: 5-Methyl-1(5
H)-phenazinone
Molecular Formula: C13H10N2O
Molecular Weight: 210.23
Percent Composition: C 74.27%, H 4.79%, N 13.33%, O 7.61%
Literature References: Blue redox pigment produced by
Pseudomonas aeruginosa. Important virulence factor in pseudomonal infection. Isoln: Wrede, Strack,
Z. Physiol. Chem. 140, 1 (1924); Schoental,
Br. J. Exp. Pathol. 22, 137 (1941). Synthesis: Wrede, Strack,
Z. Physiol. Chem. 181, 58 (1929);
Ber. 62, 2051 (1929); Surrey,
Org. Synth. 26, 86 (1946);
coll. vol. III, 753 (1955). HPLC determn in sputum: R. Wilson
et al., Infect. Immun. 56, 2515 (1988). Review of biosynthesis and role in pathogenesis: G. W. Lau
et al., Trends Mol. Med. 10, 599-606 (2004).
Properties: Dark blue needles from water (usually with 1 H2O which is lost at 50° over P2O5
in vacuo). mp 133°. Upon further heating it sublimes with decompn. Absorption spectrum: Nitzsche,
Ber. 77, 337 (1944). Freely sol in chloroform. Sol in nitrobenzene, pyridine, phenol, acetic acid, hot water, hot alcohol. Slightly sol in cold water and benzene. The blue water soln, made alkaline with Na2CO3, is easily rendered colorless by reduction with glucose or sodium hydrosulfite. Acidic KMnO4 solns are decolorized by pyocyanine. An alkaline water soln of pyocyanine acquires a maroon color upon heating.
Melting point: mp 133°