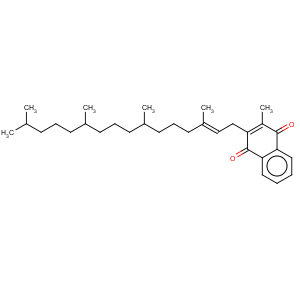
Title: Phylloquinone
CAS Registry Number: 84-80-0
CAS Name: 2-Methyl-3-[(2
E,7
R,11
R)-3,7,11,15-tetramethyl-2-hexadecenyl]-1,4-naphthalenedione
Synonyms: 2-methyl-3-phytyl-1,4-naphthoquinone; 3-phytylmenadione; phytomenadione; phytonadione; vitamin K1
Trademarks: AquaMephyton (Merck & Co.); Konakion (Roche); Mephyton (Merck & Co.); Mono-Kay (Abbott); Veda-K1 (Vedco); Veta-K1 (Sanofi)
Molecular Formula: C31H46O2
Molecular Weight: 450.70
Percent Composition: C 82.61%, H 10.29%, O 7.10%
Literature References: Photosynthetic electron carrier; occurs widely in green plants, algae, photosynthetic bacteria. Major dietary source of vitamin K,
q.v. Isoln from alfalfa: H. Dam
et al., Helv. Chim. Acta 22, 310 (1939). Structure: D. W. MacCorquodale
et al., J. Biol. Chem. 131, 357 (1939); L. F. Fieser,
J. Am. Chem. Soc. 61, 3467 (1939). Partial syntheses from menadione and phytol: H. J. Almquist, A. A. Klose,
ibid. 2557; S. B. Binkley
et al., ibid. 2558; L. F. Fieser,
ibid. 2559. Stereochemistry and total synthesis: H. Mayer
et al., Helv. Chim. Acta 47, 221 (1964); L. M. Jackman
et al., ibid. 48, 1332 (1965). Synthesis using a p-allylic nickel(I) complex: Sato
et al., J. Chem. Soc. Perkin Trans. 1 1973, 2289. Alternate synthesis: Y. Tachibana,
Chem. Lett. 1977, 901. Metabolic studies: M. J. Shearer
et al., Br. J. Haematol. 18, 297 (1970);
22, 579 (1972). The
cis isomer is not bioactive: J. T. Matschiner
et al., J. Nutr. 102, 625 (1972). Isoln from chloroplasts: E. Interschick-Niebler, H. K. Lichtenthaler,
Z. Naturforsch. 36C 276 (1981). Role in photosystem I: K. Brettel
et al., FEBS Lett. 203, 220 (1986). Conversion
in vivo to menaquinone-4,
q.v.: H. H. W. Thijssen, M. J. Drittij-Reijnders,
Br. J. Nutr. 72, 415 (1994). HPLC determn in foods: S. L. Booth
et al., J. Agric. Food Chem. 42, 295 (1994). Clinical efficacy in hemorrhagic disease of newborn: P. M. Loughnan, P. N. McDougall,
J. Paediatr. Child Health 29, 171 (1993). Comprehensive description: M. M. A. Hassan
et al., Anal. Profiles Drug Subs. 17, 449-531 (1988).
Properties: Yellow viscous oil. [a]D25 -0.28° (dioxane).
nD20 1.5263. uv max (petr ether): 242, 248, 260, 269, 325 nm (E1%1cm 396, 419, 383, 387, 68). Insol in water. Sparingly sol in methanol; sol in ethanol, acetone, benzene, petr ether, hexane, dioxane, chloroform, ether, other fat solvents and in vegetable oils. Stable to air and moisture, but dec in sunlight. Unaffected by dil acids, but destroyed by solns of alkali hydroxides and by reducing agents.
Keep well closed and protected from light.
Optical Rotation: [a]D25 -0.28° (dioxane)
Index of refraction: nD20 1.5263
Absorption maximum: uv max (petr ether): 242, 248, 260, 269, 325 nm (E1%1cm 396, 419, 383, 387, 68)
Derivative Type: Dihydro form
CAS Registry Number: 572-96-3
Synonyms: Phytonadiol; dihydrovitamin K1; a-phyllohydroquinone
Properties: Waxy mass. Freely sol in ether; sparingly sol in petr ether. Insol in water.
Derivative Type: Dihydro form sodium diphosphate
CAS Registry Number: 5988-22-7
Synonyms: Phytonadiol sodium diphosphate; Kayhydrin
Molecular Formula: C31H48Na2O8P2
Molecular Weight: 656.64
Percent Composition: C 56.70%, H 7.37%, Na 7.00%, O 19.49%, P 9.43%
Properties: mp 138°. Sol in water and methanol.
Melting point: mp 138°
Derivative Type: 2,3-Epoxide
CAS Registry Number: 25486-55-9
Synonyms: Vitamin K1 epoxide; vitamin K1 oxide
Molecular Formula: C31H46O3
Molecular Weight: 466.70
Percent Composition: C 79.78%, H 9.93%, O 10.28%
Literature References: Prepn: Fieser
et al., J. Am. Chem. Soc. 61, 3216 (1939).
Properties: Colorless oil. uv max (95% alc): 259, 305 nm (log EM 3.79, 3.31). Insol in water.
Absorption maximum: uv max (95% alc): 259, 305 nm (log EM 3.79, 3.31)
Therap-Cat: Vitamin (prothrombogenic).
Therap-Cat-Vet: Vitamin (prothrombogenic); antidote for dicoumarol poisoning.
Keywords: Vitamin/Vitamin Source; Vitamin K.