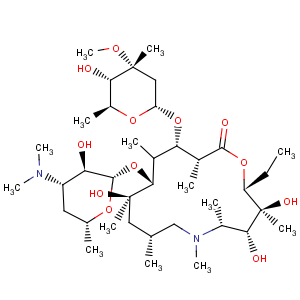
Title: Azithromycin
CAS Registry Number: 83905-01-5
CAS Name: (2
R,3
S,4
R,5
R,8
R,10
R,11
R,12
S,13
S,14
R)-13-[(2,6-Dideoxy-3-
C-methyl-3-
O-methyl-a-L-
ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-b-D-
xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one
Synonyms: N-methyl-11-aza-10-deoxo-10-dihydroerythromycin A; 9-deoxo-9a-methyl-9a-aza-9a-homoerythromycin A
Molecular Formula: C38H72N2O12
Molecular Weight: 748.98
Percent Composition: C 60.94%, H 9.69%, N 3.74%, O 25.63%
Literature References: Semi-synthetic macrolide antibiotic; related to erythromycin A,
q.v. Prepn:
BE 892357; G. Kobrehel, S. Djokic,
US 4517359 (1982, 1985 both to Sour Pliva); of the crystalline dihydrate: D. J. M. Allen, K. M. Nepveux,
EP 298650;
eidem,
US 6268489 (1989, 2001 both to Pfizer). Antibacterial spectrum: S. C. Aronoff
et al., J. Antimicrob. Chemother. 19, 275 (1987); and mode of action: J. Retsema
et al., Antimicrob. Agents Chemother. 31, 1939 (1987). Series of articles on pharmacology, pharmacokinetics, and clinical experience:
J. Antimicrob. Chemother. 31, Suppl. E, 1-198 (1993). Clinical trial in prevention of
Pneumocystis carinii pneumonia in AIDS patients: M. W. Dunne
et al., Lancet 354, 891 (1999). Review of pharmacology and clinical efficacy in pediatric infections: H. D. Langtry, J. A. Balfour,
Drugs 56, 273-297 (1998).
Properties: Amorphous solid, mp 113-115°. [a]D20 -37° (c = 1 in CHCl3).
Melting point: mp 113-115°
Optical Rotation: [a]D20 -37° (c = 1 in CHCl3)
Derivative Type: Dihydrate
CAS Registry Number: 117772-70-0
Manufacturers' Codes: CP-62993; XZ-450
Trademarks: Azitrocin (Pfizer); Ribotrex (Fabre); Sumamed (Pliva); Trozocina (Sigma-Tau); Zithromax (Pfizer); Zitromax (Pfizer)
Properties: White crystalline powder. mp 126°. [a]D26 -41.4° (c = 1 in CHCl3).
Melting point: mp 126°
Optical Rotation: [a]D26 -41.4° (c = 1 in CHCl3)
Therap-Cat: Antibacterial.
Keywords: Antibacterial (Antibiotics); Macrolides.