Title: Combretastatins
Literature References: A group of compounds isolated from the bark and stem wood of the South African bushwillow tree,
Combretum caffrum, which inhibit tubulin polmerization. The most active are combretastatins A-2 and A-4. Used in folk medicine, extracts have shown antileukemic properties. Isoln: G. R. Pettit
et al., Can. J. Chem. 60, 1374 (1982); of A-2: G. R. Pettit, S. B. Singh,
ibid. 65, 2390 (1987); of A-4:
idem et al., Experientia 45, 209 (1989). Enantioselective synthesis: A. Ramacciotti
et al., Tetrahedron: Asymmetry 7, 1101 (1996). Mechanism of action study: C. M. Lin
et al., Biochemistry 28, 6984 (1989). Antineoplastic activity of A-4: A. A. E. El-Zayat
et al., Anti-Cancer Drugs 4, 19 (1993); inhibition of tumor vascularization: G. G. Dark
et al., Cancer Res. 57, 1829 (1997). Review of structure-activity of combretastatins, podophyllotoxin,
q.v. and steganacin: D. L. Sackett,
Pharmacol. Ther. 59, 163-228 (1993).
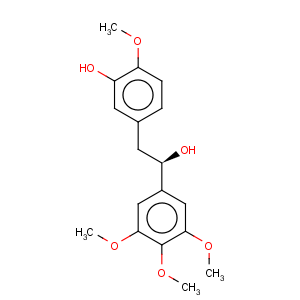
Derivative Type: Combretastatin
CAS Registry Number: 82855-09-2
CAS Name: (
R)-3-Hydroxy-4-methoxy-a-(3,4,5-trimethoxyphenyl)benzeneethanol
Synonyms: (-)-combretastatin
Molecular Formula: C18H22O6
Molecular Weight: 334.36
Percent Composition: C 64.66%, H 6.63%, O 28.71%
Properties: Needles from acetone-hexane, mp 130-131°. [a]D26 -8.51° (c = 1.41 in chloroform). uv max (methanol): 212, 228 (shoulder), 279, 287 nm (shoulder) (log e 4.33, 4.11, 3.47, 3.36). d 1.33.
Melting point: mp 130-131°
Optical Rotation: [a]D26 -8.51° (c = 1.41 in chloroform)
Absorption maximum: uv max (methanol): 212, 228 (shoulder), 279, 287 nm (shoulder) (log e 4.33, 4.11, 3.47, 3.36)
Density: d 1.33
Derivative Type: Combretastatin A-4
CAS Registry Number: 117048-59-6
CAS Name: (
Z)-2-Methoxy-5-[2-(3,4,5,-trimethoxyphenyl)ethenyl]phenol
Synonyms: 3,4,5-trimethoxy-3¢-hydroxy-4¢-methoxy-(
Z)-stilbene; CS-A4
Molecular Formula: C18H20O5
Molecular Weight: 316.35
Percent Composition: C 68.34%, H 6.37%, O 25.29%
Properties: Fine crystals from ethylacetate-hexane, mp 84.5-85.5°.
Melting point: mp 84.5-85.5°