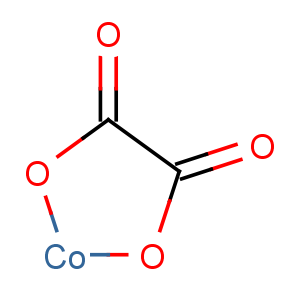
Title: Cobaltous Oxalate
CAS Registry Number: 814-89-1
Molecular Formula: C2CoO4
Molecular Weight: 146.95
Percent Composition: C 16.35%, Co 40.10%, O 43.55%
Line Formula: CoC2O4
Literature References: Prepn: Robin,
Bull. Soc. Chim. Fr. 1953, 1078;
Gmelins, Cobalt (8th ed.)
58 (part A) p 355 (1932) and supplement, pp 704-706 (1961).
Review: de Bie, Doyen,
Cobalt 15, 3-13;
16, 3-15 (1962).
Properties: d425 3.021. Readily absorbs moisture from air to form hydrates.
Density: d425 3.021
Derivative Type: Dihydrate
Properties: Light pink microcryst powder or needles. Almost insol in water; slightly sol in acids; almost insol in aq oxalic acid; freely sol in aq ammonia. Dec on heating with aq KOH or Na2CO3 soln.
Derivative Type: Tetrahydrate
Properties: Yellowish-pink amorphous powder. Effloresces on exposure to air. Loses water on heating to 100° giving the dihydrate. Very slightly sol in water; slightly sol in acids; readily sol in aq ammonia.
Use: Prepn of Co catalysts, Co metal powder for powder-metallurgical applications; stabilizer for HCN; temperature indicator.