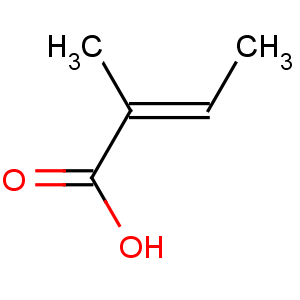
Title: Tiglic Acid
CAS Registry Number: 80-59-1
CAS Name: (2
E)-2-Methyl-2-butenoic acid
Synonyms: (
E)-2-methylcrotonic acid;
trans-2,3-dimethylacrylic acid
Molecular Formula: C5H8O2
Molecular Weight: 100.12
Percent Composition: C 59.98%, H 8.05%, O 31.96%
Literature References: The stable isomer of angelic acid. Found as glyceride in croton oil, as butyl ester in the oil of the Roman camomile,
Anthemis nobilis L.,
Compositae, and as geranyl tiglate in oil of geranium. Is formed during the charcoaling of maple wood. Formation by the intestinal roundworm,
Ascaris lumbricoides: Bueding,
J. Biol. Chem. 202, 505 (1953). Has been found in crude sodium penicillin: Cram, Tishler,
J. Am. Chem. Soc. 70, 4238 (1948). Sepn by partition chromatography: Bueding,
loc. cit. Synthesis from 2-hydroxy-2-methylbutyronitrile: Crawford,
J. Soc. Chem. Ind. London 64, 231 (1945). Review and bibliography: Buckles
et al., Chem. Rev. 55, 659-677 (1955).
Properties: Triclinic plates, rods from water. Spicy odor.
Vesicant. d 0.972. mp 63.5-64°. bp760 198.5°; bp11.5 95.0-96°. Volatile with steam.
nD81 1.4342. pK (25°) 5.02. uv max (H2O): 216-217 nm (e 10700). Molar heat of combustion 635.1 kcal. Sparingly sol in cold water; freely sol in hot water. Sol in alcohol, ether.
Melting point: mp 63.5-64°
Boiling point: bp760 198.5°; bp11.5 95.0-96°
pKa: pK (25°) 5.02
Index of refraction: nD81 1.4342
Absorption maximum: uv max (H2O): 216-217 nm (e 10700)
Density: d 0.972
Derivative Type: Calcium salt trihydrate
Molecular Formula: Ca(C5H7O2)2.3H2O
Molecular Weight: 292.34
Percent Composition: Ca 13.71%, C 41.08%, H 6.90%, O 38.31%
Properties: Leaflets. Much less sol in water than calcium angelate: 100 parts of aq soln satd at 17° contains 6.05 parts of anhydr calcium tiglate.
Derivative Type: Amide
Molecular Formula: C5H9NO
Molecular Weight: 99.13
Percent Composition: C 60.58%, H 9.15%, N 14.13%, O 16.14%
Properties: Crystals, mp 75-76°.
Melting point: mp 75-76°
Derivative Type: Methyl ester
Molecular Formula: C6H10O2
Molecular Weight: 114.14
Percent Composition: C 63.14%, H 8.83%, O 28.03%
Properties: Liquid; d420 0.9498; bp766 139.6°;
nD20 1.4370.
Boiling point: bp766 139.6°
Index of refraction: nD20 1.4370
Density: d420 0.9498
Derivative Type: Ethyl ester
Molecular Formula: C7H12O2
Molecular Weight: 128.17
Percent Composition: C 65.60%, H 9.44%, O 24.97%
Properties: Liquid; d419.5 0.9247; bp752 156°; bp11 55.5°.
nD20 1.4350. Heat of formn at constant vol: 953.2 kcal, at constant pressure: 954.4 kcal.
Boiling point: bp752 156°; bp11 55.5°
Index of refraction: nD20 1.4350
Density: d419.5 0.9247
Derivative Type: Geranyl ester
Molecular Formula: C15H24O2
Molecular Weight: 236.35
Percent Composition: C 76.23%, H 10.24%, O 13.54%
Properties: Liquid; pleasant odor; d1515 0.9279; bp7 149-151°.
Boiling point: bp7 149-151°
Density: d1515 0.9279
Use: The esters in perfumes and flavoring agents. The free acid as a breaker of emulsions.