Title: Sodium Thiosulfate
CAS Registry Number: 7772-98-7
Synonyms: Sodium hyposulfite; "hypo"; antichlor
Trademarks: Sodothiol; Sulfothiorine; Ametox
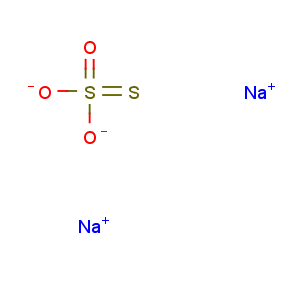
Molecular Formula: Na2O3S2
Molecular Weight: 158.11
Percent Composition: Na 29.08%, O 30.36%, S 40.56%
Line Formula: Na2S2O3
Literature References: Review of mfg processes:
Faith, Keyes & Clark's Industrial Chemicals, F. A. Lowenheim, M. K. Moran, Eds. (Wiley-Interscience, New York, 4th ed., 1975) pp 769-773. Crystal structure of the pentahydrate: A. A. Uraz, N. Armagan,
Acta Crystallogr. B33, 1396 (1977). Toxicity of the pentahydrate: C. Voegtlin
et al., J. Pharmacol. Exp. Ther. 25, 297 (1925).
Properties: Powder. Sol in water; practically insol in alcohol.
Derivative Type: Pentahydrate
Properties: Odorless crystals or granules, mp 48° when rapidly heated. Effloresces in warm dry air; slightly deliquesces in moist air. d 1.69. Loses all its water at 100°; dec at higher temp. Sol in water; insol in alcohol. Slowly dec in aq soln at ordinary temp, more rapidly when heated. The aq soln is practically neutral. pH 6.5-8.0. It dissolves silver halides and many other salts of silver.
Incompat: Iodine, acids; lead, mercury, and silver salts. LD i.v. in rats: >2.5 g/kg (Voegtlin).
Melting point: mp 48° when rapidly heated
Density: d 1.69
Toxicity data: LD i.v. in rats: >2.5 g/kg (Voegtlin)
Use: To remove chlorine from solns according to the eq: Na2S2O3 + 4Cl2 +5H2O ? 2NaHSO4 + 8HCl and Na2S2O3 + 2HCl ? 2NaCl + H2O + S + SO2. As "antichlor" in bleaching of paper pulp; as fixer in photography; for extraction of silver from ores; as mordant in dyeing and printing textiles; reducer in chrome dyeing; manuf leather; bleaching bone, straw, ivory; also as reagent in analytical chemistry.
Therap-Cat: Antidote (cyanide).
Therap-Cat-Vet: Antidote (cyanide). Has been used as a "general detoxifier", in bloat, and externally in ringworm, mange.
Keywords: Antidote (Cyanide).