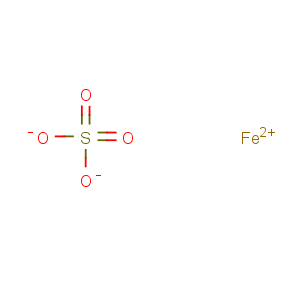Title: Ferrous Sulfate
CAS Registry Number: 7720-78-7
Molecular Formula: FeO4S
Molecular Weight: 151.91
Percent Composition: Fe 36.76%, O 42.13%, S 21.11%
Line Formula: FeSO4
Literature References: Hydrates occur in nature as the minerals:
melanterite,
siderotil,
szomolnikite,
tauriscite. Heptahydrate prepd commercially by the action of H2SO4 on Fe:
Faith, Keyes & Clark's Industrial Chemicals, F. A. Lowenheim, M. K. Moran, Eds. (Wiley-Interscience, New York, 4th ed., 1975) pp 418-421. Crystal structure of heptahydrate: Baur,
Acta Crystallogr. 17, 1167 (1964). Acute toxicity: Hoppe
et al., Am. J. Med. Sci. 230, 491 (1955).
Derivative Type: Monohydrate
CAS Registry Number: 17375-41-6
Synonyms: Dried ferrous sulfate; exsiccated ferrous sulfate
Trademarks: Feromax; Feroritard (Nikken); Ferro-Gradumet (Abbott); Fespan (SK & F); Tetucur (Teikoku Zoki)
Properties: White to yellow cryst powder. Loses H2O at about 300°. Dec at higher temps. Sol in water.
Derivative Type: Heptahydrate
CAS Registry Number: 7782-63-0
Synonyms: Copperas; green vitriol; iron vitriol
Trademarks: Feosol (SK & F); Feospan (SK & F); Fesofor (SK & F); Fero-Gradumet (Abbott); Fer-in-Sol (Mead Johnson); Haemofort; Ironate (Wyeth); Mol-Iron (Schering); Presfersul; Sulferrous (Conal)
Properties: Blue-green, monoclinic, odorless crystals or granules. Efflorescent in dry air; oxidizes in moist air forming a brown coating of basic ferric sulfate. Forms tetrahydrate at 56.6° and monohydrate at 65°. d 1.897. Sol in water. Practically insol in alcohol. Aq solns are oxidized slowly by air when cold, rapidly when hot; rate of oxidation increased by addn of alkali or exposure to light. LD50 in mice: 65 mg/kg i.v.; 1.52 g/kg orally (Hoppe).
Density: d 1.897
Toxicity data: LD50 in mice: 65 mg/kg i.v.; 1.52 g/kg orally (Hoppe)
CAUTION: Potential symptoms of overexposure are irritation of eyes, skin, mucous membranes; GI disturbances, abdominal pain, vomiting, diarrhea; dehydration; shock, pallor, cyanosis, coldness; rapid, weak pulse; low blood pressure; rapid, shallow respirations; drowsiness; hyporeflexia; dilated pupils; coma; liver damage.
See NIOSH Pocket Guide to Chemical Hazards (DHHS/NIOSH 97-140, 1997) p 174;
Clinical Toxicology of Commercial Products, R. E. Gosselin
et al., Eds. (Williams & Wilkins, Baltimore, 5th ed., 1984) Sect. III, pp 179-185.
Use: In manufacture of Fe, Fe compds, other sulfates; in Fe electroplating baths; in fertilizer; as food and feed supplement; in radiation dosimeters; as reducing agent in chemical processes; as wood preservative; as weed-killer; in prevention of chlorosis in plants; in other pesticides; in writing ink; in process engraving and lithography; as dye for leather; in etching aluminum; in water treatment; in qualitative analysis ("brown ring" test for nitrates); as polymerization catalyst.
Therap-Cat: Hematinic.
Therap-Cat-Vet: In iron deficiency. Astringent.
Keywords: Hematinic.