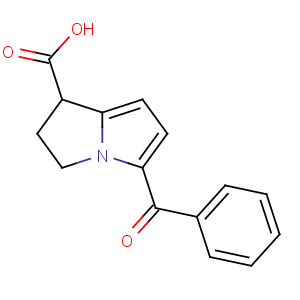
Title: Ketorolac
CAS Registry Number: 74103-06-3
CAS Name: 5-Benzoyl-2,3-dihydro-1
H-pyrrolizine-1-carboxylic acid
Synonyms: 5-benzoyl-1,2-dihydro-3
H-pyrrolo[1,2-a]pyrrole-1-carboxylic acid
Manufacturers' Codes: RS-37619
Molecular Formula: C15H13NO3
Molecular Weight: 255.27
Percent Composition: C 70.58%, H 5.13%, N 5.49%, O 18.80%
Literature References: Prostaglandin biosynthesis inhibitor. Prepn and separation of isomers:
BE 856681; J. M. Muchowski, A. F. Kluge,
US 4089969 (both 1978 to Syntex). Alternate processes: J. M. Muchowski, R. Greenhouse,
US 4347186 (1982 to Syntex); F. Franco
et al., J. Org. Chem. 47, 1682 (1982); J. B. Doherty,
US 4496741 (1985 to Merck & Co.). Absolute configuration: A. Guzman
et al., J. Med. Chem. 29, 589 (1986). Structure-activity relationships: J. M. Muchowski
et al., ibid. 28, 1037 (1985). Pharmacology and analgesic, anti-inflammatory profile of ketorolac and its tromethamine salt: W. H. Rooks
et al., Agents Actions 12, 684 (1982);
eidem, Drugs Exp. Clin. Res. 11, 479 (1985). Clinical comparison with acetaminophen in post-operative pain: H. J. McQuay
et al., Clin. Pharmacol. Ther. 39, 89 (1986).
Properties: Crystals from ethyl acetate + ether, mp 160-161°. uv max in methanol: 245, 312 nm (e 7080, 17400). pKa 3.49 ±0.02. LD50 orally in mice: ~200 mg/kg (Rooks).
Melting point: mp 160-161°
pKa: pKa 3.49 ±0.02
Absorption maximum: uv max in methanol: 245, 312 nm (e 7080, 17400)
Toxicity data: LD50 orally in mice: ~200 mg/kg (Rooks)
Derivative Type: (±)-Form tromethamine salt
CAS Registry Number: 74103-07-4
Trademarks: Acular (Allergan); Dolac (Syntex); Lixidol (Farmitalia); Tarasyn (Syntex); Toradol (Syntex); Toratex (Syntex)
Molecular Formula: C19H24N2O6
Molecular Weight: 376.40
Percent Composition: C 60.63%, H 6.43%, N 7.44%, O 25.50%
Derivative Type: (+)-Form
Properties: Crystals from hexane + ethyl acetate, mp 174° (Guzman); also reported as mp 154-156° (Muchowski, Kluge). [a]D +173° (c = 1 in methanol).
Melting point: mp 174° (Guzman); mp 154-156° (Muchowski, Kluge)
Optical Rotation: [a]D +173° (c = 1 in methanol)
Derivative Type: (-)-Form
Properties: Crystals from hexane + ethyl acetate, mp 169-170° (Guzman); also reported as mp 153-155° (Muchowski, Kluge). [a]D -176° (c = 1 in methanol).
Melting point: mp 169-170° (Guzman); mp 153-155° (Muchowski, Kluge)
Optical Rotation: [a]D -176° (c = 1 in methanol)
Therap-Cat: Analgesic; anti-inflammatory.
Keywords: Analgesic (Non-Narcotic); Anti-inflammatory (Nonsteroidal); Arylcarboxylic Acids.