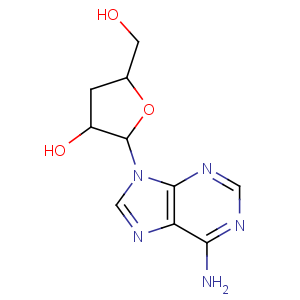
Title: Cordycepin
CAS Registry Number: 73-03-0
CAS Name: 3¢-Deoxyadenosine
Synonyms: 9-cordyceposidoadenine
Molecular Formula: C10H13N5O3
Molecular Weight: 251.24
Percent Composition: C 47.81%, H 5.22%, N 27.88%, O 19.10%
Literature References: First reported nucleoside antibiotic. Isoln from culture fluids of
Cordyceps militaris (Linn.) Link: K. G. Cunningham
et al., J. Chem. Soc. 1951, 2299; N. M. Kredich, A. J. Guarino,
Biochim. Biophys. Acta 41, 363 (1960). Proposed structure: H. R. Bentley
et al., J. Chem. Soc. 1951, 2301. Identity with 3¢-deoxyadenosine and revised structure: E. A. Kaczka
et al., Biochem. Biophys. Res. Commun. 14, 456 (1964). Biosynthesis: R. Suhadolnik
et al., J. Am. Chem. Soc. 86, 948 (1964). Synthesis: A. R. Todd, T. L. Ulbricht,
J. Chem. Soc. 1960, 3275; W. W. Lee
et al., J. Am. Chem. Soc. 83, 1906 (1961); E. Walton
et al., ibid. 86, 2952 (1964); Y. Ito
et al., ibid. 103, 6739 (1981). Cordycepin and cordycepin triphosphate have been used extensively in the study of messenger RNA transcription,
see H. T. Shigeura, G. E. Boxer,
Biochem. Biophys. Res. Commun. 17, 758 (1964); S. Penman
et al., Proc. Natl. Acad. Sci. USA 67, 1878 (1970).
Reviews: J. J. Fox.
et al., "Nucleoside Antibiotics" in
Prog. Nucleic Acid Res. Mol. Biol. 5, 258-262 (1966); A. J. Guarino, "Cordycepin" in
Antibiotics I, D. Gottlieb, P. Shaw, Eds. (Springer-Verlag, New York, 1967) pp 468-480.
Properties: Needles from ethanol,
n-butanol,
n-propanol or water. mp 225-226°. [a]D20 -47°. [a]D27 -42°. uv max (ethanol): 260 nm (e 14600). pH aq soln: 7.1.
Melting point: mp 225-226°
Optical Rotation: [a]D20 -47°; [a]D27 -42°
Absorption maximum: uv max (ethanol): 260 nm (e 14600)
Derivative Type: Triphosphate
Synonyms: Cordycepin-5¢-triphosphate; 3¢-deoxy ATP; 3¢-deoxyadenosine-5¢-(tetrahydrogen triphosphate)
Molecular Formula: C10H13N5O12P3
Molecular Weight: 488.16
Percent Composition: C 24.60%, H 2.68%, N 14.35%, O 39.33%, P 19.04%
Literature References: Formation by conversion of 3¢-deoxyadenosine in Ehrlich ascites tumor: H. Klenow,
Biochim. Biophys. Acta 76, 347 (1963). Metabolism in KB cells: H. Shigeura, S. Sampson,
ibid. 138, 26 (1967). Synthesis: J. J. Novak, F. Sorm,
Collect. Czech. Chem. Commun. 38, 113 (1973); M. Blandin,
J. Carbohyd. Nucl., Nucl. 3(5/6), 341 (1976).
Properties: Inhibits the final step of RNA biosynthesis by termination of the ribonucleotide chain due to the absence of the 3¢-hydroxyl group.