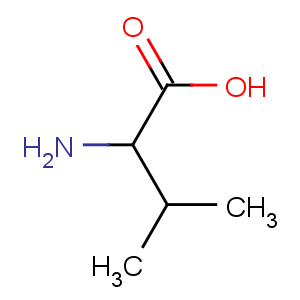
Title: Valine
CAS Registry Number: 72-18-4
CAS Name: L-Valine
Synonyms: Val; V; 2-aminoisovaleric acid; 2-amino-3-methylbutyric acid; a-aminoisovaleric acid; (
S)-2-amino-3-methylbutanoic acid
Molecular Formula: C5H11NO2
Molecular Weight: 117.15
Percent Composition: C 51.26%, H 9.46%, N 11.96%, O 27.31%
Literature References: An essential amino acid for human development. Identified from organ extracts in 1856 by von Group-Besanez; isolated from proteins in 1879 by Schützenberger who proposed that it was aminovaleric acid. Structure confirmation: E. Fischer,
Ber. 39, 2320 (1906). Early chemistry and biochemistry:
Amino Acids and Proteins, D. M. Greenberg, Ed. (Charles C. Thomas, Springfield, IL, 1951) 950 pp.,
passim; J. P. Greenstein, M. Winitz,
Chemistry of the Amino Acids vols 1-3 (John Wiley and Sons, Inc., New York, 1961) pp. 2368-2380,
passim. Conformation study: R. H. Yun, J. Hermans,
Protein Eng. 4, 761 (1991). Nutritional assessment study: V. R. Young
et al., J. Nutr. 102, 1159 (1972); in parenteral nutrition: P. Reiderer
et al., Nutr. Metab. 24, 209 (1980); in hemodialysis patients: G. A. Young
et al., Kidney Int. 21, 492 (1982). Brief review of metabolism: P. Kamoun,
Trends Biochem. Sci. 17, 175-176 (1992).
Properties: Leaflets from water + alcohol, mp 315° (closed capillary). d 1.230. Sublimes. [M]D +33.1° (5
N HCl); +72.6° (glacial acetic acid); [a]D23 +22.9° (c = 0.8 in 20% HCl). pK1 2.32; pK2 9.62. Soly in water at 0°: 83.4 g/l; at 25°: 88.5 g/l; at 50°: 96.2 g/l; at 65°: 102.4 g/l. Insol in common neutral solvents.
Melting point: mp 315° (closed capillary)
pKa: pK1 2.32; pK2 9.62
Optical Rotation: [a]D23 +22.9° (c = 0.8 in 20% HCl)
Density: d 1.230
Derivative Type: DL-Form
Properties: Sublimes without melting at ordinary speed of heating. Dec 298° (closed capillary, very rapid heating). One part dissolves in 11.7 parts of water at 15°, in 14.1 parts of water at 25°. Insol in common neutral solvents.