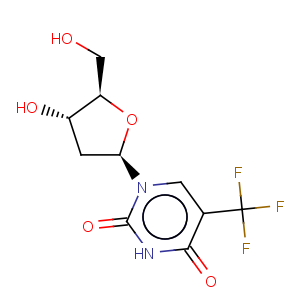
Title: Trifluridine
CAS Registry Number: 70-00-8
CAS Name: a,a,a-Trifluorothymidine
Synonyms: 2¢-deoxy-5-(trifluoromethyl)uridine; 5-(trifluoromethyl)-2¢-deoxyuridine; F3TDR
Manufacturers' Codes: NSC-75520
Trademarks: TFT Thilo (Alcon-Thilo); Virophta (Dulcis); Viroptic (Burroughs Wellcome)
Molecular Formula: C10H11F3N2O5
Molecular Weight: 296.20
Percent Composition: C 40.55%, H 3.74%, F 19.24%, N 9.46%, O 27.01%
Literature References: Prepn: C. Heidelberger
et al., J. Am. Chem. Soc. 84, 3597 (1962);
eidem, J. Med. Chem. 7, 1 (1964); C. Heidelberger,
US 3201387 (1965 to U.S. Dept. HEW). Crystal structure: A. H. Tench,
Diss. Abstr. Int. B 33, 3587 (1973). NMR study: R. J. Cushley
et al., J. Am. Chem. Soc. 90, 709 (1968). Metabolism: D. L. Dexter
et al., Cancer Res. 32, 247 (1972); W. J. O'Brien, H. F. Edelhauser,
Invest. Ophthalmol. Visual Sci. 16, 1093 (1977). Pharmacodynamics: B. L. Wigdahl, J. R. Parkhurst,
Antimicrob. Agents Chemother. 14, 470 (1978); G. J. Smith
et al., Biochem. Biophys. Res. Commun. 83, 1538 (1978). Teratogenicity study: M. Itoi
et al., Arch. Ophthalmol. 93, 46 (1975). Cytotoxicity and mutagenicity study: E. Huberman, C. Heidelberger,
Mutat. Res. 14, 130 (1972). Clinical studies: H. E. Kaufman,
Invest. Ophthalmol. Visual Sci. 17, 941 (1978); R. A. Hyndiuk
et al., Arch. Ophthalmol. 96, 1839 (1978). Review of mechanism of antiviral activity: C. Heidelberger,
Ann. N.Y. Acad. Sci. 255, 317 (1975). Review of pharmacology and therapeutic use: A. A. Carmine
et al., Drugs 23, 329-353 (1982).
Properties: Cryst from ethyl acetate, mp 186-189°. uv max (0.1
N HCl): 260 nm (e 9960); (0.1
N NaOH): 260 nm (e 6590).
Melting point: mp 186-189°
Absorption maximum: uv max (0.1
N HCl): 260 nm (e 9960); (0.1
N NaOH): 260 nm (e 6590)
Therap-Cat: Antiviral (ophthalmic).
Keywords: Antiviral; Purines/Pyrimidinones.