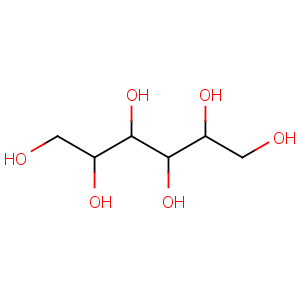
Title: Mannitol
CAS Registry Number: 69-65-8
Synonyms: D-Mannitol; mannite; manna sugar; cordycepic acid
Trademarks: Manicol; Mannidex; Osmitrol (Baxter); Osmosal; Resectisol (McGaw)
Molecular Formula: C6H14O6
Molecular Weight: 182.17
Percent Composition: C 39.56%, H 7.75%, O 52.70%
Literature References: Widespread in plants and plant exudates; obtained from manna and seaweeds:
The Carbohydrates, W. Pigman, Ed. (Academic Press, New York, 1957) pp 249-250. Forms a stable, equimolar compound with H2O2: S. Tanatar,
J. Russ. Phys. Chem. Soc. 40, 376,
C.A. 3, 883 (1909). Prepd by electrolytic reduction of glucose: Creighton,
Can. Chem. Process Ind. 26, 690 (1942),
C.A. 37, 10885 (1943); Wolfrom
et al., J. Am. Chem. Soc. 68, 578 (1946). Prepn from seaweed: Sorensen, Kristensen,
US 2516350 (1950); by hydrogenation of invert sugar, monosaccharides, and sucrose: Kasehagen, and Kasehagen, Luskin,
US 2642462,
US 2749371, and
US 2759024 (1953, 1956, and 1956, all to Atlas Powder). Review of prepn: Pigman,
loc. cit. Novel synthesis: M. Makkee
et al., Chem. Commun. 1980, 930.
Properties: Orthorhombic needles from alc, mp 166-168°. Sweetish taste. d20 1.52. bp3.5 290-295°. Is inactive or very slightly levorotatory in distilled water. Forms sodium mannitoborate on addition of borax giving a greater rotation: [a]D20 +23° ? +24° after 1 hr in a soln of 10 g mannitol +12.8 g borax + sufficient H2O to make 100 ml. One gram dissolves in ~5.5 ml water, 83 ml alcohol; more sol in hot water. Insol in ether. Sol in pyridine, aniline, aq solns of alkalies. One gram dissolves in 18 ml glycerol (d 1.24). Soly tables: Creighton, Klauder,
J. Franklin Inst. 195, 687 (1923). pKa (18°): 13.50.
Melting point: mp 166-168°
Boiling point: bp3.5 290-295°
pKa: pKa (18°): 13.50
Optical Rotation: [a]D20 +23° ? +24° after 1 hr in a soln of 10 g mannitol +12.8 g borax + sufficient H2O to make 100 ml
Density: d20 1.52; d 1.24
Use: Used with boric acid in the manuf of dry electrolytic condensers for radio applications; in making artificial resins and plasticizers; in pharmacy as excipient and diluent for solids and liqs; in analytical chemistry for boron determinations; in the manuf of mannitol hexanitrate. Used in the food industry as anticaking and free-flow agent, flavoring agent, lubricant and release agent, stabilizer and thickener and nutritive sweetener.
Therap-Cat: Diuretic. Diagnostic aid (renal function).
Keywords: Diagnostic Aid; Diuretic.