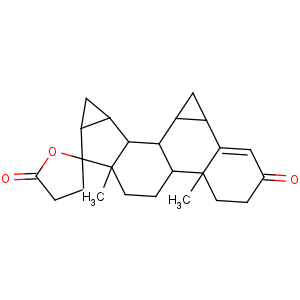
Title: Drospirenone
CAS Registry Number: 67392-87-4
CAS Name: (2¢
S,6
R,7
R,8
R,9
S,10
R,13
S,14
S,15
S,16
S)-1,3¢,4¢,6,7,8,9,10,11,12,13,14,15,16,20,21-Hexadecahydro-10,13-dimethylspiro[17
H-dicyclopropa[6,7:15,16]cyclopenta[
a]phenanthrene-17,2¢(5¢
H)-furan]-3,5¢(2
H)-dione
Synonyms: 6b,7b,15b,16b-dimethylene-3-oxo-4-androstene-[17(b-1¢)-spiro-5¢]perhydrofuran-2¢-one; 6b,7b,15b,16b-dimethylen-3-oxo-17a-pregn-4-ene-21,17-carbolactone; dihydrospirorenone
Manufacturers' Codes: ZK-30595
Molecular Formula: C24H30O3
Molecular Weight: 366.49
Percent Composition: C 78.65%, H 8.25%, O 13.10%
Literature References: Synthetic progestogen exhibiting antimineralocorticoid and antiandrogenic activity. Prepn: R. Wiechert
et al., DE 2652761;
eidem, US 4129564 (both 1978 to Schering AG); D. Bittler
et al., Angew. Chem. 94, 718 (1982). HPLC determn in human plasma: W. Krause, U. Jakobs,
J. Chromatogr. 230, 37 (1982). Pharmacological profile: P. Muhn
et al., Contraception 51, 99 (1995). Review of synthesis: H. Laurent
et al., J. Steroid Biochem. 19, 771-776 (1983); of pharmacology and clinical experience: W. Oelkers,
Mol. Cell. Endocrinol. 217, 255-261 (2004).
Properties: mp 201.3°. [a]D22 -182° (c = 0.5 in chloroform). uv (methanol): 265 nm (e 19000).
Melting point: mp 201.3°
Optical Rotation: [a]D22 -182° (c = 0.5 in chloroform)
Derivative Type: Mixture with ethinyl estradiol
Trademarks: Angeliq (Schering AG); Yasmin (Schering AG)
Literature References: Clinical trial as oral contraceptive: K. S. Parsey, A. Pong,
Contraception 61, 105 (2000); in treatment of menopausal symptoms: R. Schürmann
et al., Climacteric 7, 189 (2004).
Therap-Cat: Progestogen. In combination with estrogen as oral contaceptive and in treatment of menopausal symptoms.
Keywords: Progestogen; Contraceptive (Oral).