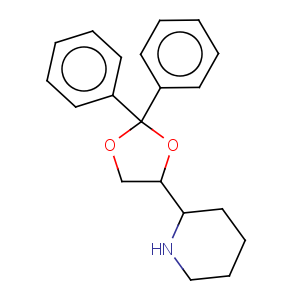
Title: Dioxadrol
CAS Registry Number: 6495-46-1
CAS Name: 2-(2,2-Diphenyl-1,3-dioxolan-4-yl)piperidine
Synonyms: 2,2-diphenyl-4-(2-piperidyl)-1,3-dioxolane
Molecular Formula: C20H23NO2
Molecular Weight: 309.40
Percent Composition: C 77.64%, H 7.49%, N 4.53%, O 10.34%
Literature References: Prepn of
dl-forms and resolution of a-racemates: Hardie, Halverstadt,
BE 613262;
US 3262938 (1962, 1966 both to Cutter Labs.); W. R. Hardie
et al., J. Med. Chem. 9, 127 (1966).
Properties: Oily liquid.
Derivative Type: Hydrochloride
CAS Registry Number: 3666-69-1
Trademarks: Rydar (Cutter)
Molecular Formula: C20H23NO2.HCl
Molecular Weight: 345.86
Percent Composition: C 69.45%, H 6.99%, N 4.05%, O 9.25%, Cl 10.25%
Properties: Crystals from methanol, mp 256-260°. LD50 orally in mice: 240 mg/kg (Hardie).
Melting point: mp 256-260°
Toxicity data: LD50 orally in mice: 240 mg/kg (Hardie)
Derivative Type: d-Form hydrochloride
CAS Registry Number: 1162-15-8
Synonyms: Dexoxadrol hydrochloride
Trademarks: Relane (Cutter)
Properties: Crystals, dec 254°. [a]D20 +34° (c = 2 in methanol). LD50 orally in mice: 340 mg/kg (Hardie).
Optical Rotation: [a]D20 +34° (c = 2 in methanol)
Toxicity data: LD50 orally in mice: 340 mg/kg (Hardie)
Derivative Type: l-Form hydrochloride
Synonyms: Levoxadrol hydrochloride
Trademarks: Levoxan (Cutter)
Properties: Crystals, mp 248-254°. [a]D20 -34.5° (c = 2 in methanol). LD50 orally in mice: 230 mg/kg (Hardie).
Melting point: mp 248-254°
Optical Rotation: [a]D20 -34.5° (c = 2 in methanol)
Toxicity data: LD50 orally in mice: 230 mg/kg (Hardie)
Therap-Cat: Base as antidepressant;
d-form hydrochloride as stimulant (central), analgesic;
l-form hydrochloride as anesthetic (local), relaxant (smooth muscle).
Keywords: Antidepressant.