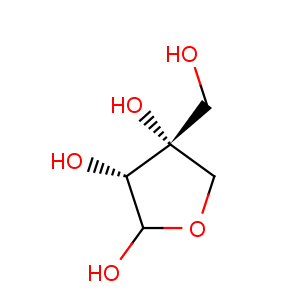
Title: Apiose
CAS Registry Number: 639-97-4
CAS Name: D-Apiose
Synonyms: tetrahydroxyisovaleraldehyde; 3-
C-(hydroxymethyl)-D-glyceroaldotetrose
Molecular Formula: C5H10O5
Molecular Weight: 150.13
Percent Composition: C 40.00%, H 6.71%, O 53.29%
Literature References: First found in parsley in which it occurs as the flavinoid glycoside apiin,
q.v. Isoln from apiin: Vongerichten,
Ann. 318, 126 (1901);
321, 74 (1902); Hemming, Ollis,
Chem. Ind. (London) 1953, 85. From the rubber plant,
Hevea brasiliensis, Euphorbiaceae: Patrick,
Nature 178, 216 (1956). Discussion of structure and isoln from the Australian marine plant
Posidonia australis Kon.,
Potamogetonaceae: Bell,
Methods in Carbohydrate Chemistry vol. I (Academic Press, New York, 1962) pp 260-263. Synthesis: Gorin, Perlin,
Can. J. Chem. 36, 480 (1958); Khalique,
J. Chem. Soc. 1962, 2515; Ezekiel
et al., Tetrahedron Lett. 1969, 1635. Synthesis of L-form: Weygand, Schmiechen,
Ber. 92, 535 (1959); of DL-form: Kinoshita, Miwa,
Carbohydr. Res. 28, 175 (1973); Y. Araki
et al., ibid. 58, C4 (1977); of D- and L-forms: P. Ho,
Can. J. Chem. 57, 381 (1979). Chemistry, configuration and synthesis studies: Williams, Jones,
ibid. 42, 69 (1964); Hulyalker
et al., ibid. 43, 2085 (1965).
Review: Watson, Orenstein,
Adv. Carbohydr. Chem. Biochem. 31, 135-184 (1975).
Properties: Syrup. [a]D15 +5.6°; [a]D19 +9.1°. Soluble in water.
Optical Rotation: [a]D15 +5.6°; [a]D19 +9.1°
Derivative Type: D-Apiose di-
O-isopropylidene
Molecular Formula: C11H18O5
Molecular Weight: 230.26
Percent Composition: C 57.38%, H 7.88%, O 34.74%
Properties: Plates from water containing a trace of NH3, mp 81-83°. [a]D20 +55.5° (c = 1.1 in ethanol).
Melting point: mp 81-83°
Optical Rotation: [a]D20 +55.5° (c = 1.1 in ethanol)