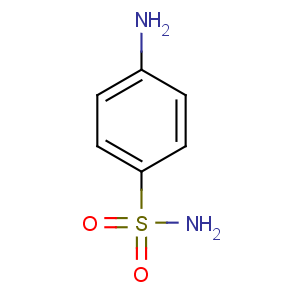
Title: Sulfanilamide
CAS Registry Number: 63-74-1
CAS Name: 4-Aminobenzenesulfonamide
Synonyms: p-anilinesulfonamide;
p-sulfamidoaniline
Manufacturers' Codes: 1162-F
Trademarks: Prontosil album; Prontylin; Streptocide (Frosst)
Molecular Formula: C6H8N2O2S
Molecular Weight: 172.20
Percent Composition: C 41.85%, H 4.68%, N 16.27%, O 18.58%, S 18.62%
Literature References: Active metabolite of the antibacterial dye, sulfamidochrysoidine,
q.v. Prepn: P. Gelmo,
J. Prakt. Chem. 77, 369 (1908); A. Galat,
Ind. Eng. Chem. 36, 192 (1944); Hurdis, Yang,
J. Chem. Educ. 46, 697 (1969). Large-scale process: F. Mietzsch, J. Klarer,
US 2132178;
eidem et al., US 2276664 (1938, 1942 both to Winthrop). Antibacterial activity: J. Tréfouel
et al., C.R. Seances Soc. Biol. Ses Fil. 120, 756 (1935); and clinical efficacy: L. Colebrook, A. W. Purdie,
Lancet 233, 1291 (1937). Toxicity study: E. K. Marshall, Jr.
et al., J. Am. Med. Assoc. 110, 252 (1938). Historical review: M. H. Bickel,
Gesnerus 45, 67-86 (1988).
Properties: Crystals from boiling water, mp 164.5-166.5°. Neutral to litmus. pH (0.5% aq soln): 5.8-6.1. uv max: 255, 312 nm. One gram dissolves in about 37 ml alcohol; in about 5 ml acetone; in about 2 ml boiling water. Sol in glycerol, HCl, solns of K and Na hydroxides. Practically insol in chloroform, ether, benzene. LD50 orally in mice: 3.8 g/kg (Marshall).
Melting point: mp 164.5-166.5°
Absorption maximum: uv max: 255, 312 nm
Toxicity data: LD50 orally in mice: 3.8 g/kg (Marshall)
Derivative Type: N4-Acetylsulfanilamide
CAS Registry Number: 121-61-9
CAS Name: N-[4-(Aminosulfonyl)phenyl]acetamide
Molecular Formula: C8H10N2O3S
Molecular Weight: 214.24
Percent Composition: C 44.85%, H 4.70%, N 13.08%, O 22.40%, S 14.97%
Literature References: Major metabolite of sulfanilamide: E. K. Marshall, Jr.
et al., Science 85, 202 (1937).
Properties: Needles from water, mp 219°.
Melting point: mp 219°
Therap-Cat: Antibacterial.
Therap-Cat-Vet: Antibacterial.
Keywords: Antibacterial (Synthetic); Sulfonamides.