Title: Cefoperazone
CAS Registry Number: 62893-19-0
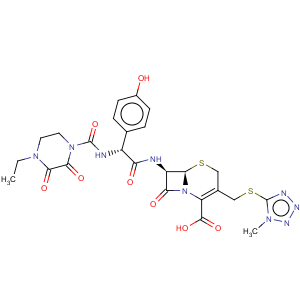
CAS Name: (6
R,7
R)-7-[[(2
R)-[[(4-Ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino](4-hydroxyphenyl)acetyl]amino]-3-[[(1-methyl-1
H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
Synonyms: 7-[D-(-)-a-(4-ethyl-2,3-dioxo-1-piperazinecarboxamido)-a-(4-hydroxyphenyl)acetamido]-3-[[(1-methyl-1
H-tetrazol-5-yl)thio]methyl]-3-cephem-4-carboxylic acid
Molecular Formula: C25H27N9O8S2
Molecular Weight: 645.67
Percent Composition: C 46.50%, H 4.21%, N 19.52%, O 19.82%, S 9.93%
Literature References: Broad spectrum third generation cephalosporin antibiotic. Prepn: I. Saikawa
et al., BE 837682;
eidem, US 4410522 (1976, 1983 both to Toyama);
eidem, Yakugaku Zasshi 99, 929 (1979). Stability in aq soln:
eidem, ibid. 1207.
In vitro activity: M. V. Borobio
et al., Antimicrob. Agents Chemother. 17, 129 (1980). Kinetics in rats: J. Fabre
et al., Schweiz. Med. Wochenschr. 110, 264 (1980); in humans: A. F. Allaz,
ibid. 109, 1999 (1979). Review of pharmacology and therapeutic efficacy: R. N. Brogden
et al., Drugs 22, 423-460 (1981). Symposium on clinical studies:
ibid. Suppl. 1, 1-124.
Properties: Crystals from acetonitrile/water, mp 169-171° (hydrated). Stable at pH 4.0-7.0; slightly unstable in acid; highly unstable in alkaline soln.
Melting point: mp 169-171° (hydrated)
Derivative Type: Sodium salt
CAS Registry Number: 62893-20-3
Manufacturers' Codes: CP-52640-2; T-1551
Trademarks: Bioperazone (Biopharma); Cefazone (Firma); Cefobid (Pfizer); Cefobine (Pfizer); Cefobis (Pfizer); Cefogram (Metapharma); Cefoneg (Tosi); Cefosint (Proter); Dardum (Lisapharma); Farecef (Lafare); Kefazon (Esseti); Novobiocyl (Francia); Pathozone (Pfizer); Peracef (Pfizer); Perocef (Pulitzer); Tomabef (Aandersen)
Molecular Formula: C25H26N9NaO8S2
Molecular Weight: 667.65
Percent Composition: C 44.97%, H 3.93%, N 18.88%, Na 3.44%, O 19.17%, S 9.61%
Therap-Cat: Antibacterial.
Therap-Cat-Vet: Antibacterial.
Keywords: Antibacterial (Antibiotics); ?Lactams; Cephalosporins.