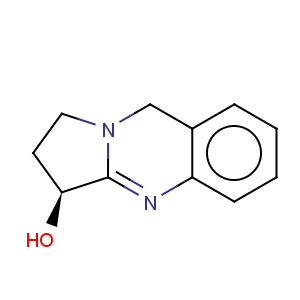
Title: Vasicine
CAS Registry Number: 6159-55-3
CAS Name: 1,2,3,9-Tetrahydropyrrolo[2,1-
b]quinazolin-3-ol
Synonyms: peganine
Molecular Formula: C11H12N2O
Molecular Weight: 188.23
Percent Composition: C 70.19%, H 6.43%, N 14.88%, O 8.50%
Literature References: Isoln from
Adhatoda vasica Nees,
Acanthaceae: Hooper,
Pharm. J. 18, 841 (1888); Sen, Ghose,
J. Indian Chem. Soc. 1, 315 (1924); Mehta
et al., J. Org. Chem. 28, 445 (1963). Isoln from
Peganum harmala L.,
Zygophyllaceae: Sp?th, Nikawitz,
Ber. 67, 45 (1934); Sp?th, Kuffner,
ibid. 67, 868 (1934). Structure and synthesis: Sp?th
et al., ibid. 68, 699 (1935); Sp?th, Platzer,
ibid. 69, 255 (1936). Synthesis of
dl-vasicine: Southwick, Casanova,
J. Am. Chem. Soc. 80, 1168 (1958).
Reviews: Sp?th,
Monatsh. Chem. 72, 115 (1938); H. T. Openshaw, "The Quinazoline Alkaloids" in
The Alkaloids vol. III, R. H. F. Manske, H. L. Holmes, Eds. (Academic Press, New York, 1953) pp 101-118; Ray,
J. Indian Chem. Soc. 35, 697 (1958).
Derivative Type: dl-Form
Properties: Needles from alc. mp 210°. Sublimes in high vacuum. Sol in acetone, alcohol, chloroform; slightly sol in water, ether, benzene.
Melting point: mp 210°
Derivative Type: l-Form
Properties: Needles from alc, mp 212°. [a]D14 -254° (c = 2.4 in CHCl3); [a]D14 -62° (c = 2.4 in alc). In dil HCl this alkaloid is dextrorotatory.
Melting point: mp 212°
Optical Rotation: [a]D14 -254° (c = 2.4 in CHCl3); [a]D14 -62° (c = 2.4 in alc)
Derivative Type: Hydrochloride dihydrate
Properties: Needles, mp 208° (dry).
Melting point: mp 208° (dry)
Derivative Type: Hydriodide dihydrate
Properties: Needles, mp 195° (dry).
Melting point: mp 195° (dry)
Derivative Type: Methiodide
Properties: Needles from methanol, mp 187°.
Melting point: mp 187°
Derivative Type: Acetylvasicine
Molecular Formula: C11H11N2OCOCH3
Molecular Weight: 230.26
Percent Composition: C 67.81%, H 6.13%, N 12.17%, O 13.90%
Properties: Crystals, mp 123°, bp0.01 230-240°.
Melting point: mp 123°
Boiling point: bp0.01 230-240°