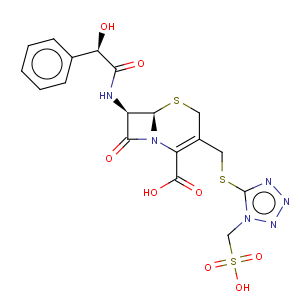
Title: Cefonicid
CAS Registry Number: 61270-58-4
CAS Name: (6
R,7
R)-7-[[(2
R)-Hydroxyphenylacetyl]amino]-8-oxo-3-[[[1-(sulfomethyl)-1
H-tetrazol-5-yl]thio]methyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
Synonyms: (6
R,7
R)-7-[(
R)-mandelamido]-8-oxo-3-[[[1-(sulfomethyl)-1
H-tetrazol-5-yl]thio]methyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
Molecular Formula: C18H18N6O8S3
Molecular Weight: 542.57
Percent Composition: C 39.85%, H 3.34%, N 15.49%, O 23.59%, S 17.73%
Literature References: Injectable semi-synthetic cephalosporin antibiotic related to cefamandole,
q.v. Prepn: D. A. Berges,
DE 2611270;
idem, US 4048311 (1976, 1977 both to Smith Kline);
US 4093723,
US 4159373 (1978, 1979 both to Smith Kline). Antibacterial activity, pharmacokinetics: P. Actor
et al., Antimicrob. Agents Chemother. 13, 784 (1978). Series of articles on stability, comparative
in vitro activity, serum levels:
Curr. Chemother. Infect. Dis., Proc. 11th Int. Congr. Chemother. vol. 1 (Am. Soc. Microbiol., Washington, D.C., 1979) pp 246-254. Stability towards b-lactamases: R. Mehta
et al., J. Antibiot. 34, 202 (1981). Kinetics and renal handling: D. Pitkin
et al., Clin. Pharmacol. Ther. 30, 587 (1981).
In vitro evaluation vs Group B streptococci: A. S. Bayer
et al., Antimicrob. Agents Chemother. 21, 344 (1982). Review of antibacterial activity, pharmacology, therapeutic use: E. Saltiel, R. N. Brogden,
Drugs 32, 222-259 (1986).
Derivative Type: Disodium salt
CAS Registry Number: 61270-78-8
Manufacturers' Codes: SKF-75073
Trademarks: Cefodie (SKB); Monocid (SKB); Monocidur (SKB); Praticef (Zambon)
Molecular Formula: C18H16N6Na2O8S3
Molecular Weight: 586.53
Percent Composition: C 36.86%, H 2.75%, N 14.33%, Na 7.84%, O 21.82%, S 16.40%
Therap-Cat: Antibacterial.
Keywords: Antibacterial (Antibiotics); ?Lactams; Cephalosporins.