Title: e-Aminocaproic Acid
CAS Registry Number: 60-32-2
CAS Name: 6-Aminohexanoic acid
Synonyms: epsilon-aminocaproic acid; epsilcapramin; EACA
Manufacturers' Codes: CY-116
Trademarks: Amicar (Wyeth); Epsikapron (Pharmacia); Hemocaprol (Sanofi-Aventis)
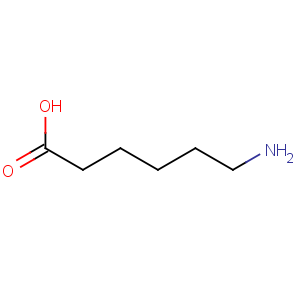
Molecular Formula: C6H13NO2
Molecular Weight: 131.17
Percent Composition: C 54.94%, H 9.99%, N 10.68%, O 24.39%
Line Formula: H2N(CH2)5COOH
Literature References: Antifibrinolytic; inhibits the activity of plasmin and plasminogen,
q.q.v. Prepn: S. Gabriel, T. A. Maass,
Ber. 32, 1266 (1899); A. Galat, S. Mallin,
J. Am. Chem. Soc. 68, 2729 (1946); C. Y. Meyers, L. E. Miller,
Org. Synth. coll. vol. IV, 39 (1963). Toxicity data: D. W. Hallesy
et al., Pharmacologist 3, 62 (1961). Crystal structure: G. Bodor
et al., Acta Crystallogr. 23, 482 (1967). Clinical trial and mode of action study: T. J. Vander Salm
et al., J. Thorac. Cardiovasc. Surg. 112, 1098 (1996).
Properties: Crystals from alcohol, mp 202-203°. pK1 4.43; pK2 10.75. Freely sol in water; sparingly in methanol. Practically insol in ethanol, chloroform. LD50 in rats (g/kg): 7.0 i.p.; ~3.3 i.v. (Hallesy).
Melting point: mp 202-203°
pKa: pK1 4.43; pK2 10.75
Toxicity data: LD50 in rats (g/kg): 7.0 i.p.; ~3.3 i.v. (Hallesy)
Derivative Type: Hydrochloride
CAS Registry Number: 4321-58-8
Molecular Formula: C6H13NO2.HCl
Molecular Weight: 167.63
Percent Composition: C 42.99%, H 8.42%, N 8.36%, O 19.09%, Cl 21.15%
Properties: mp 128-129°.
Melting point: mp 128-129°
Therap-Cat: Hemostatic.
Keywords: Hemostatic.