Title: Ubenimex
CAS Registry Number: 58970-76-6
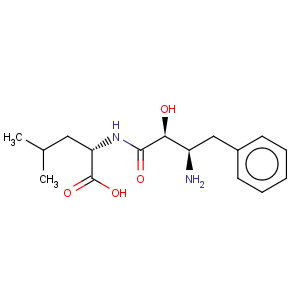
CAS Name: N-[(2
S,3
R)-3-Amino-2-hydroxy-1-oxo-4-phenylbutyl]-L-leucine
Manufacturers' Codes: NK-421
Trademarks: Bestatin (Nippon Kayaku)
Molecular Formula: C16H24N2O4
Molecular Weight: 308.37
Percent Composition: C 62.32%, H 7.84%, N 9.08%, O 20.75%
Literature References: Dipeptide antitumor antibiotic produced by
Streptomyces olivoreticuli with immunostimulant activity; inhibits leucine aminopeptidase and aminopeptidases B and N. Prepn from fermentation broth: H. Umezawa
et al., DE 2528984;
eidem, US 4052449 (1976, 1977 both to Microbiochem. Res. Found., Japan);
eidem, J. Antibiot. 29, 97 (1976). Structure: H. Suda
et al., ibid. 100. Synthesis of stereoisomers and structure-activity study: R. Nishizawa
et al., J. Med. Chem. 20, 510 (1977). Stereocontrolled synthesis: S. Kobayashi
et al., Tetrahedron Lett. 25, 5079 (1984). Crystal structure: J. S. Ricci, Jr.
et al., J. Org. Chem. 47, 3063 (1982). Cell surface binding studies: H. Umezawa
et al., J. Antibiot. 29, 857 (1976); W. E. Müller
et al., Int. J. Immunopharmacol. 4, 393 (1982). Acute toxicity: T. Sakakibara
et al., Jpn. J. Antibiot. 36, 2971 (1983). Review of pharmacology: G. Mathé,
Biomed. Pharmacother. 45, 49-54 (1991); of clinical studies: K. Ota,
ibid. 55-60. Clinical trial in squamous-cell lung carcinoma: Y. Ichinose
et al., J. Natl. Cancer Inst. 95, 605 (2003).
Properties: Colorless needles, mp 233-236°. [a]D20 -15.5° (c = 1.0 in 1
N HCl). pKa 8.1, 3.1. uv max: 241.5, 248, 253, 258, 264.5, 268 nm (E1%1cm 3.8, 4.0, 5.0, 6.0, 4.6, 2.7). Sol in acetic acid, DMSO, methanol. Less sol in water. Insol in ethyl acetate, benzene, hexane, chloroform. LD50 in male, female mice, male, female rats (g/kg): 1.3, 1.9, 1.9, 2.1 s.c.; 0.19, 0.19, 0.90, 0.78 i.p.; >4.0, >4.0, >2.0, >2.0 orally (Sakakibara).
Melting point: mp 233-236°
pKa: pKa 8.1, 3.1
Optical Rotation: [a]D20 -15.5° (c = 1.0 in 1
N HCl)
Absorption maximum: uv max: 241.5, 248, 253, 258, 264.5, 268 nm (E1%1cm 3.8, 4.0, 5.0, 6.0, 4.6, 2.7)
Toxicity data: LD50 in male, female mice, male, female rats (g/kg): 1.3, 1.9, 1.9, 2.1 s.c.; 0.19, 0.19, 0.90, 0.78 i.p.; >4.0, >4.0, >2.0, >2.0 orally (Sakakibara)
Therap-Cat: Immunomodulator; antineoplastic.
Keywords: Antineoplastic; Immunomodulators; Immunomodulator.