References of 1-Phenanthrenecarboxylicacid, 7-ethenyl-1,2,3,4,4a,4b,5,6,7,8,10,10a-dodecahydro-1,4a,7-trimethyl-,(1R,4aR,4bS,7S,10aR)-
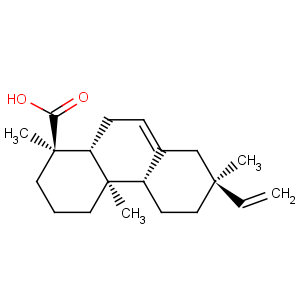
Title: Isopimaric Acid
CAS Registry Number: 5835-26-7
CAS Name: [1
R-(1a,4ab,4ba,7a,10aa)]-7-Ethenyl-1,2,3,4,4a,4b,5,6,7,8,10,10a-dodecahydro-1,4a,7-trimethyl-1-phenanthrenecarboxylic acid
Synonyms: 13b-methyl-13-vinylpodocarp-7-ene-15-oic acid; miropinic acid
Molecular Formula: C20H30O2
Molecular Weight: 302.45
Percent Composition: C 79.42%, H 10.00%, O 10.58%
Literature References: Isoln from
Dacrydium biforme Pilg.,
Podocarpaceae: Hosking, Brandt,
Ber. 68, 1311 (1935); from the bled resin of
Podocarpus ferrugineus, Podocarpaceae: Brandt, Neubauer,
J. Chem. Soc. 1940, 683; from
Pimus palustris Mill.,
Pinaceae: Harris, Sanderson,
J. Am. Chem. Soc. 70, 2079, 2081 (1948). Identity of isopimaric and miropinic acids: Brossi, Jeger,
Helv. Chim. Acta 33, 722 (1950). Structure: Antkowiak
et al., J. Org. Chem. 27, 1930 (1962). Stereochemistry: Ireland, Newbould,
ibid. 1931; Antkowiak
et al., Can. J. Chem. 43, 1257 (1965); Bose
et al., Indian J. Chem. 5, 228 (1967).
Properties: Needles from methanol or ethanol, mp 160°. [a]D16 -3.6° (10.4% soln in 1:1 alcohol:chloroform). Freely sol in chloroform, benzene; moderately sol in ether, alcohol; slightly sol in light petroleum. Absorption spectrum: Brossi, Jeger,
loc. cit.
Melting point: mp 160°
Optical Rotation: [a]D16 -3.6° (10.4% soln in 1:1 alcohol:chloroform)